Deposition Date
2007-08-09
Release Date
2007-11-20
Last Version Date
2024-05-29
Entry Detail
PDB ID:
2Z6U
Keywords:
Title:
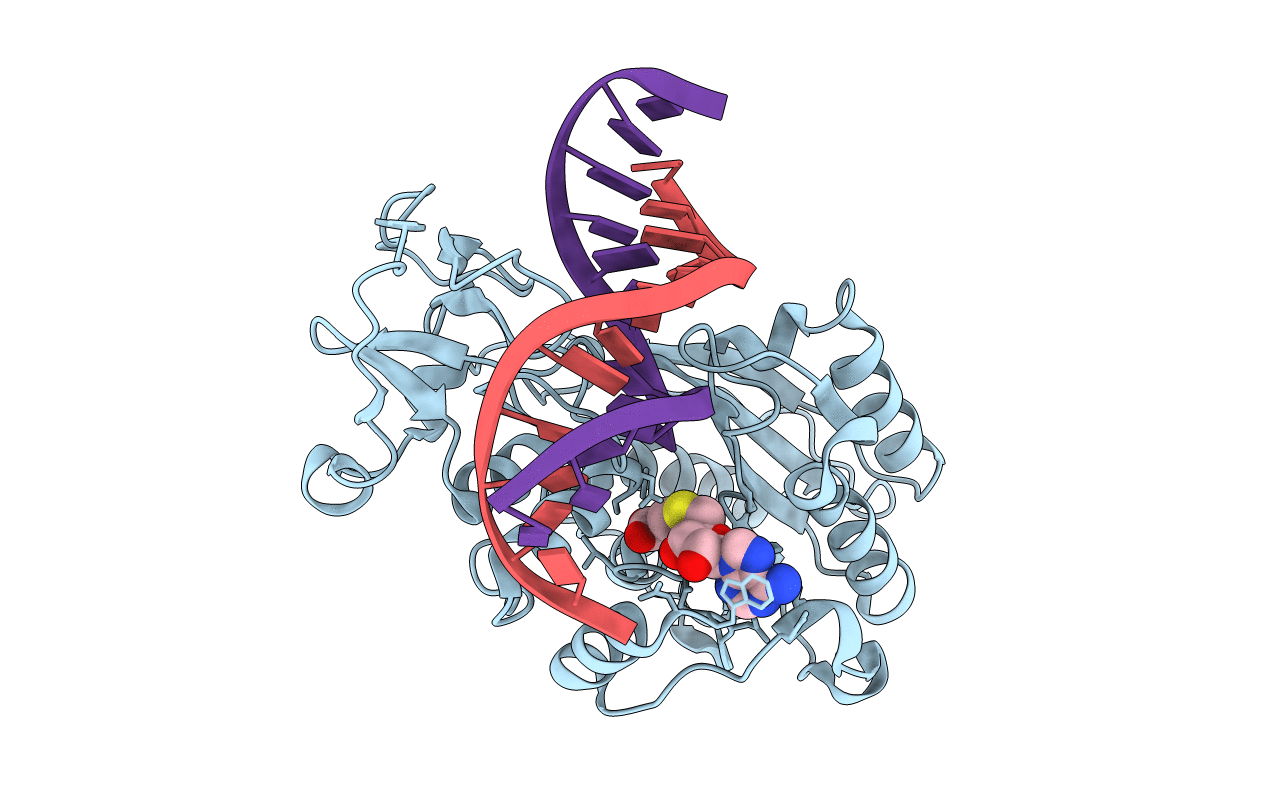
Ternary structure of the Glu119Ala M.HhaI, C5-Cytosine DNA methyltransferase, with unmodified DNA and AdoHcy
Biological Source:
Source Organism(s):
Haemophilus parahaemolyticus (Taxon ID: 735)
Expression System(s):
Method Details:
Experimental Method:
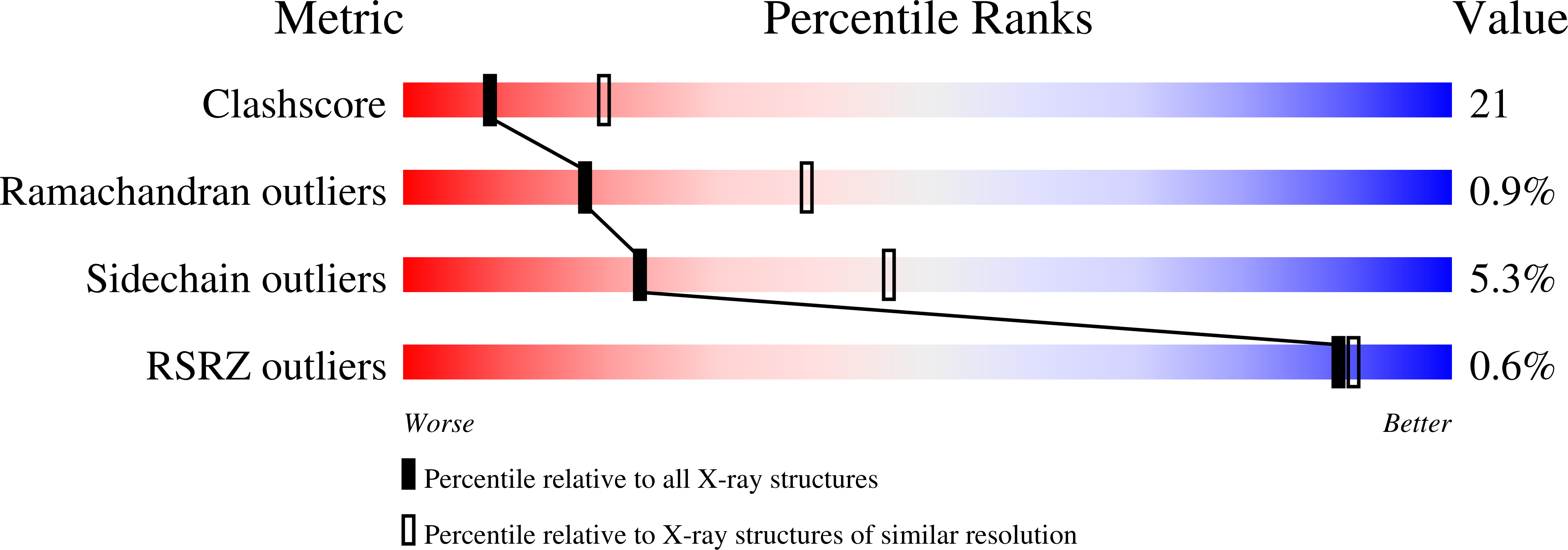
Resolution:
2.72 Å
R-Value Free:
0.26
R-Value Work:
0.21
Space Group:
H 3 2