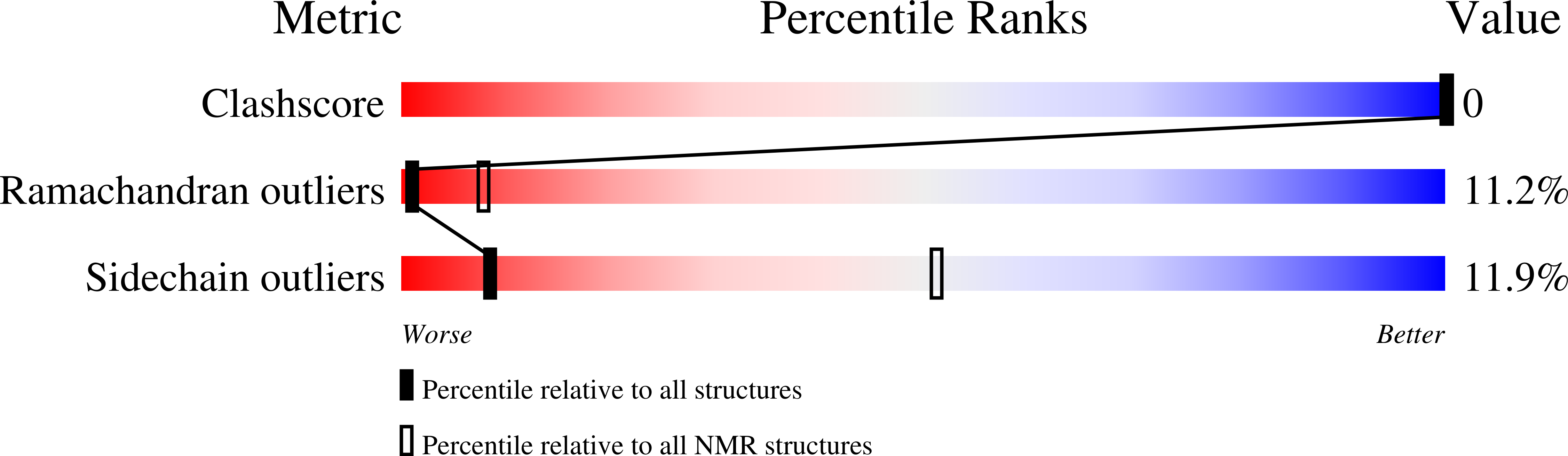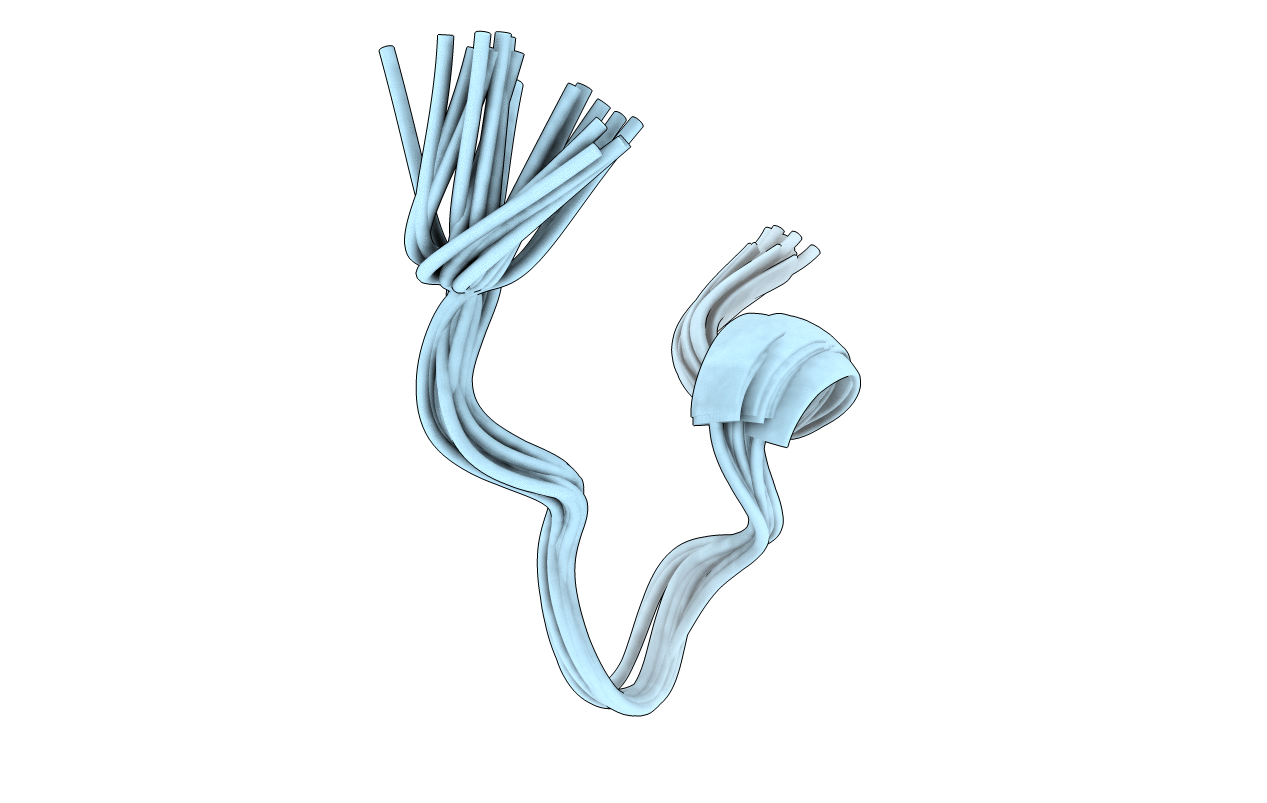
Deposition Date
2007-04-29
Release Date
2008-04-08
Last Version Date
2022-03-16
Entry Detail
PDB ID:
2YYF
Keywords:
Title:
Purification and structural characterization of a D-amino acid containing conopeptide, marmophine, from Conus marmoreus
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
target function