Deposition Date
2007-04-26
Release Date
2008-04-29
Last Version Date
2023-10-25
Entry Detail
PDB ID:
2YXN
Keywords:
Title:
Structual basis of azido-tyrosine recognition by engineered bacterial Tyrosyl-tRNA synthetase
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 316407)
Expression System(s):
Method Details:
Experimental Method:
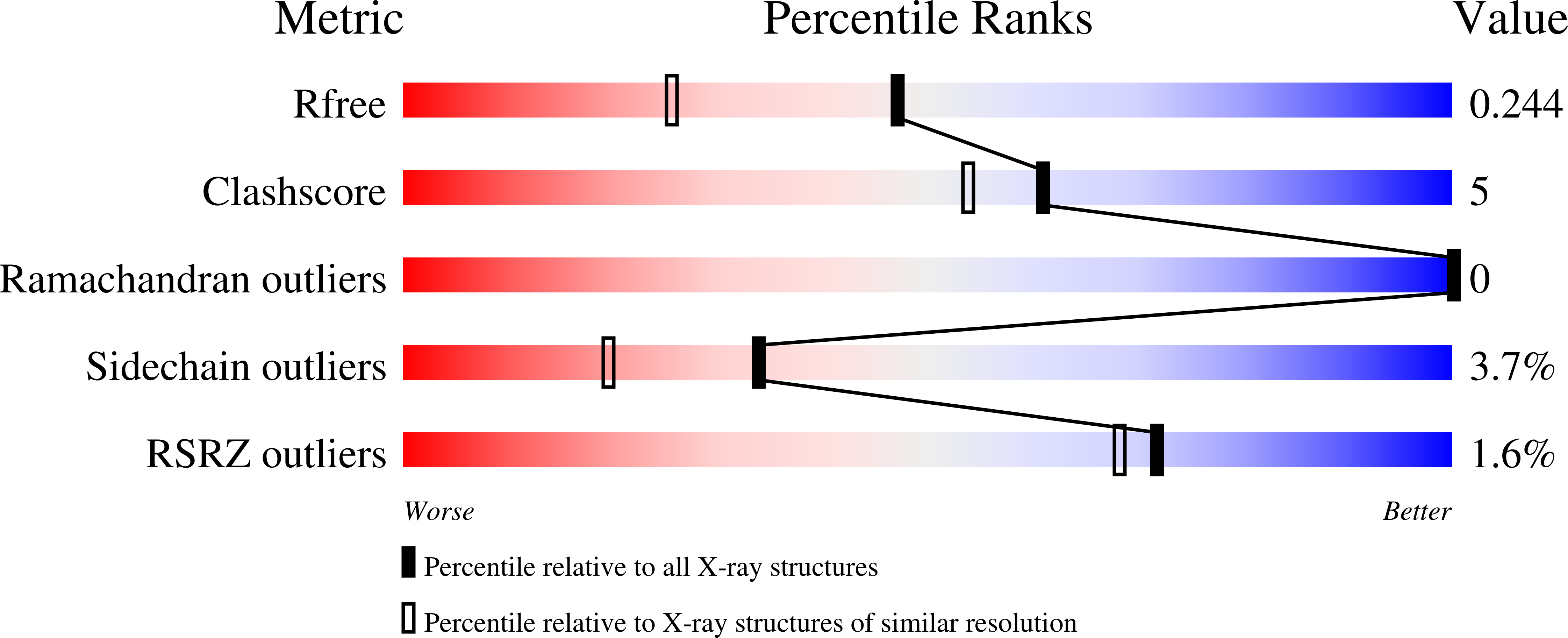
Resolution:
1.80 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 31 2 1