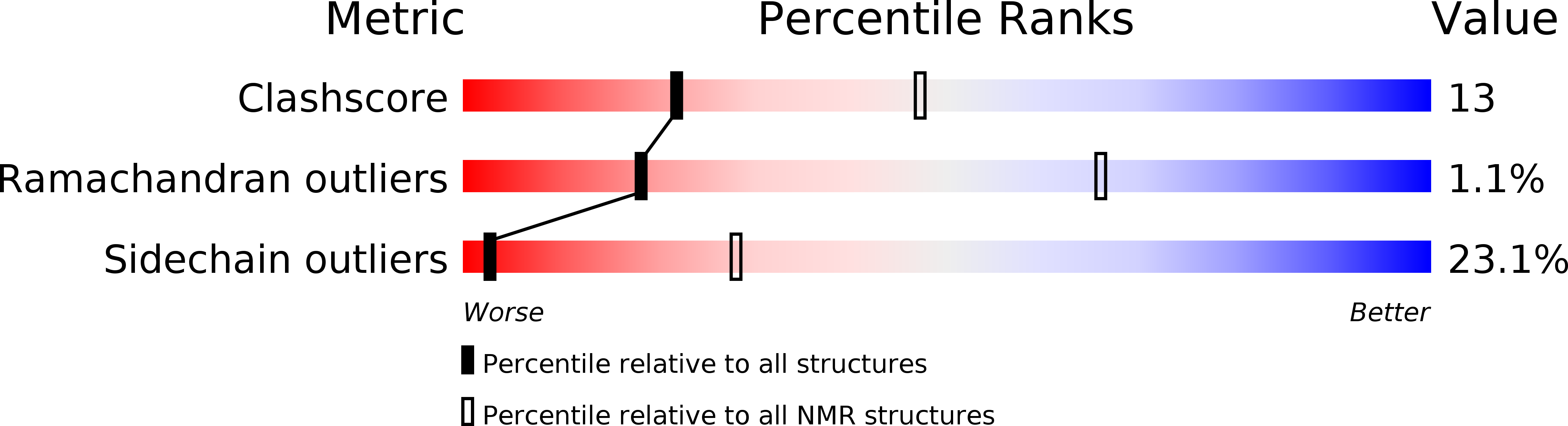
Deposition Date
2011-05-26
Release Date
2012-06-13
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2YKA
Keywords:
Title:
RRM domain of mRNA export adaptor REF2-I bound to HVS ORF57 peptide
Biological Source:
Source Organism(s):
MUS MUSCULUS (Taxon ID: 10090)
SAIMIRIINE HERPESVIRUS 2 (Taxon ID: 10381)
SAIMIRIINE HERPESVIRUS 2 (Taxon ID: 10381)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy