Deposition Date
2011-03-08
Release Date
2011-06-01
Last Version Date
2025-12-10
Entry Detail
PDB ID:
2YBO
Keywords:
Title:
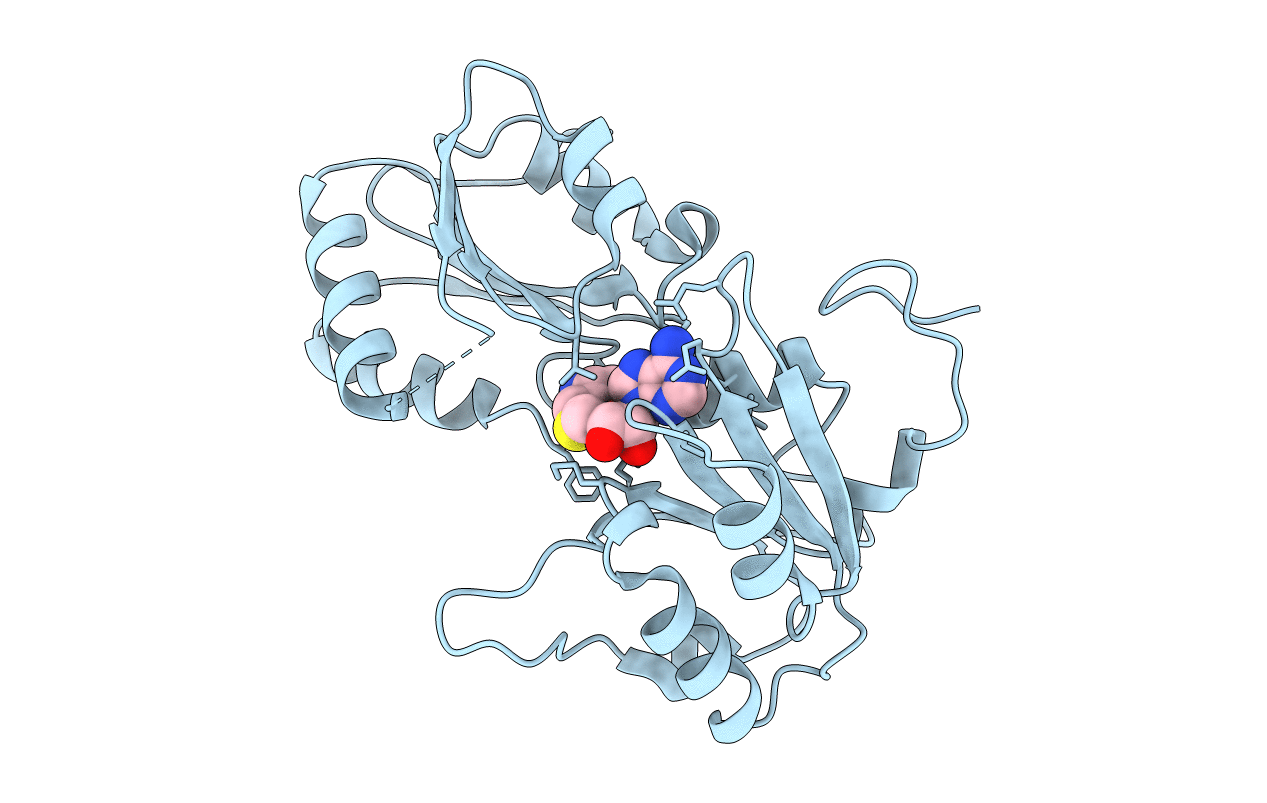
The x-ray structure of the SAM-dependent uroporphyrinogen III methyltransferase NirE from Pseudomonas aeruginosa in complex with SAH
Biological Source:
Source Organism(s):
PSEUDOMONAS AERUGINOSA (Taxon ID: 208964)
Expression System(s):
Method Details:
Experimental Method:
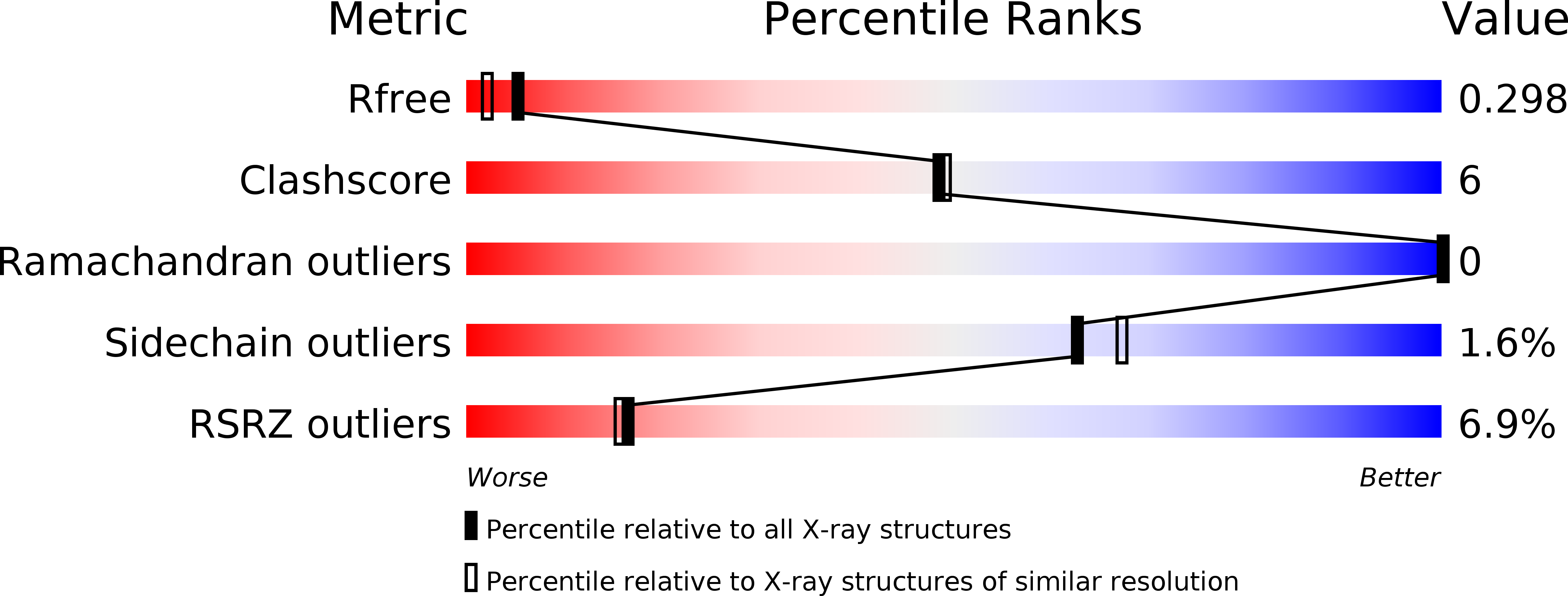
Resolution:
2.00 Å
R-Value Free:
0.28
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
C 2 2 21