Deposition Date
2011-03-08
Release Date
2011-07-20
Last Version Date
2024-10-23
Entry Detail
PDB ID:
2YBJ
Keywords:
Title:
Nitrate X-ray induced reduction on HEWL crystals (12.31 MGy)
Biological Source:
Source Organism(s):
GALLUS GALLUS (Taxon ID: 9031)
Method Details:
Experimental Method:
Resolution:
2.00 Å
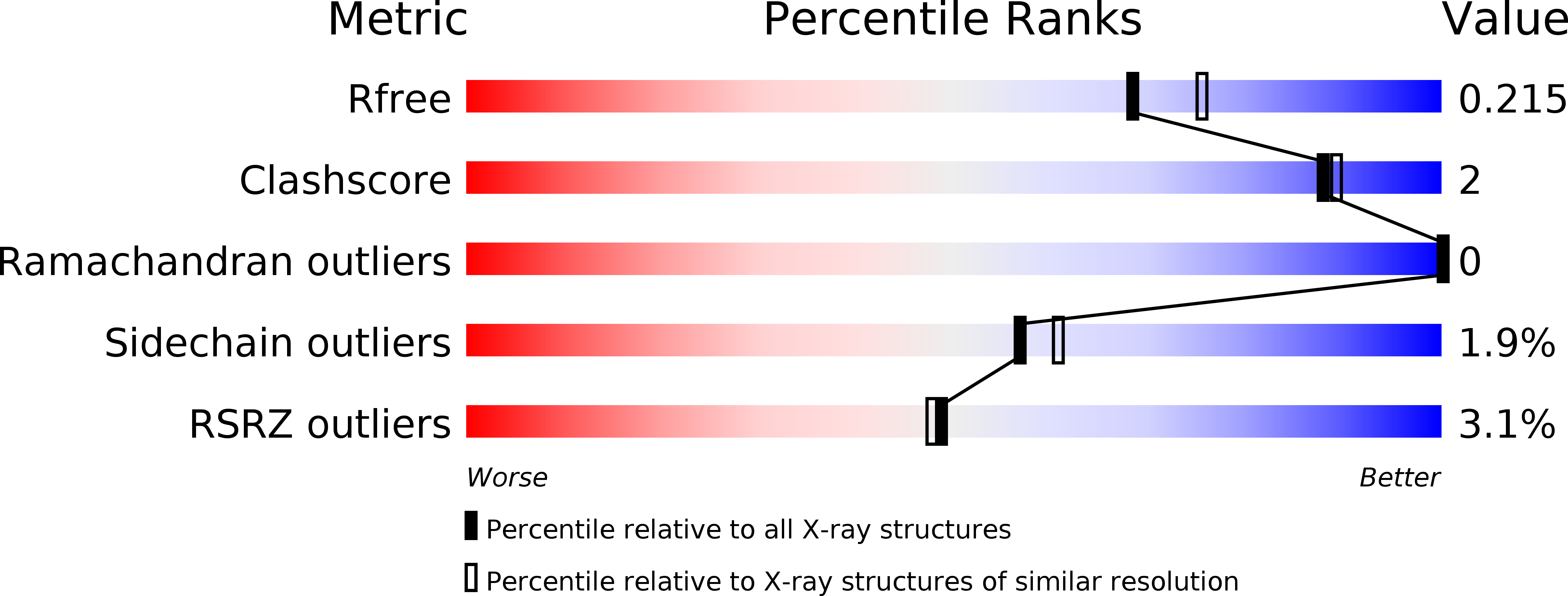
R-Value Free:
0.21
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 43 21 2