Deposition Date
2010-11-25
Release Date
2011-10-05
Last Version Date
2023-12-20
Entry Detail
PDB ID:
2XZF
Keywords:
Title:
CRYSTAL STRUCTURE OF A COMPLEX BETWEEN THE WILD-TYPE LACTOCOCCUS LACTIS FPG (MUTM) AND AN OXIDIZED PYRIMIDINE CONTAINING DNA AT 293K
Biological Source:
Source Organism(s):
LACTOCOCCUS LACTIS SUBSP. CREMORIS (Taxon ID: 1359)
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
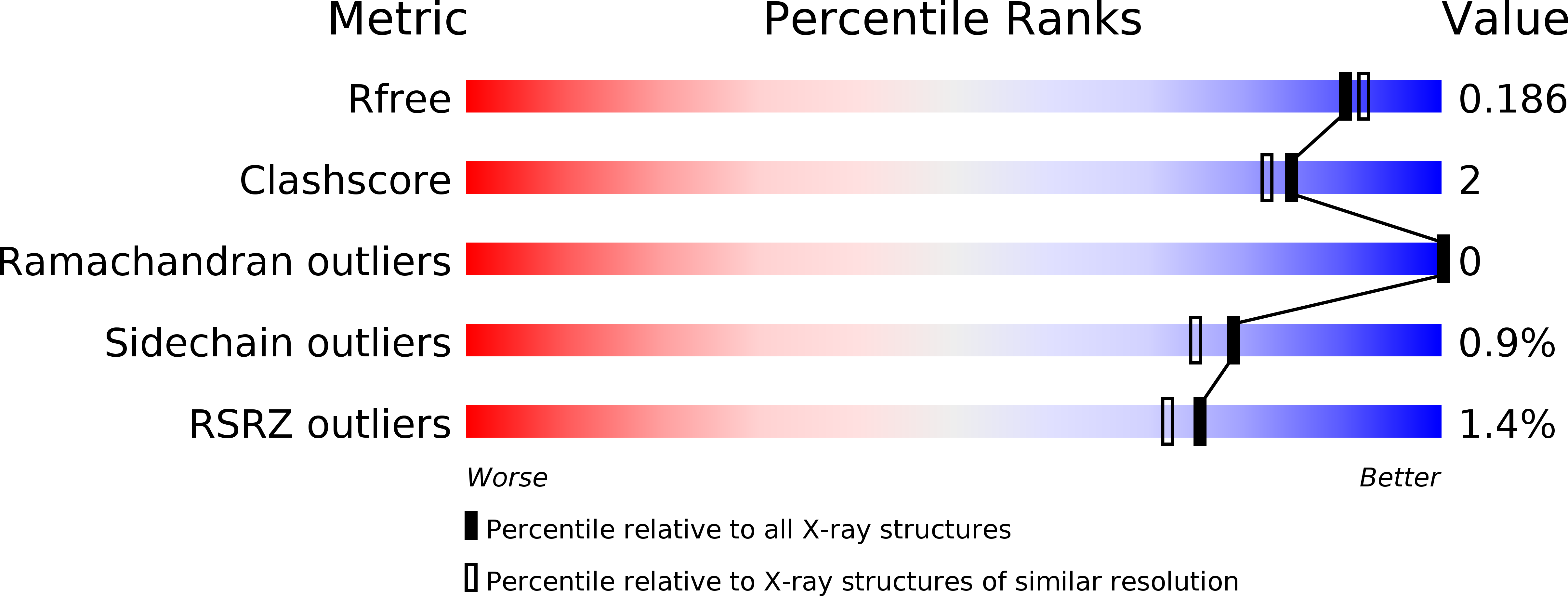
Resolution:
1.80 Å
R-Value Free:
0.19
R-Value Work:
0.15
R-Value Observed:
0.16
Space Group:
P 41 21 2