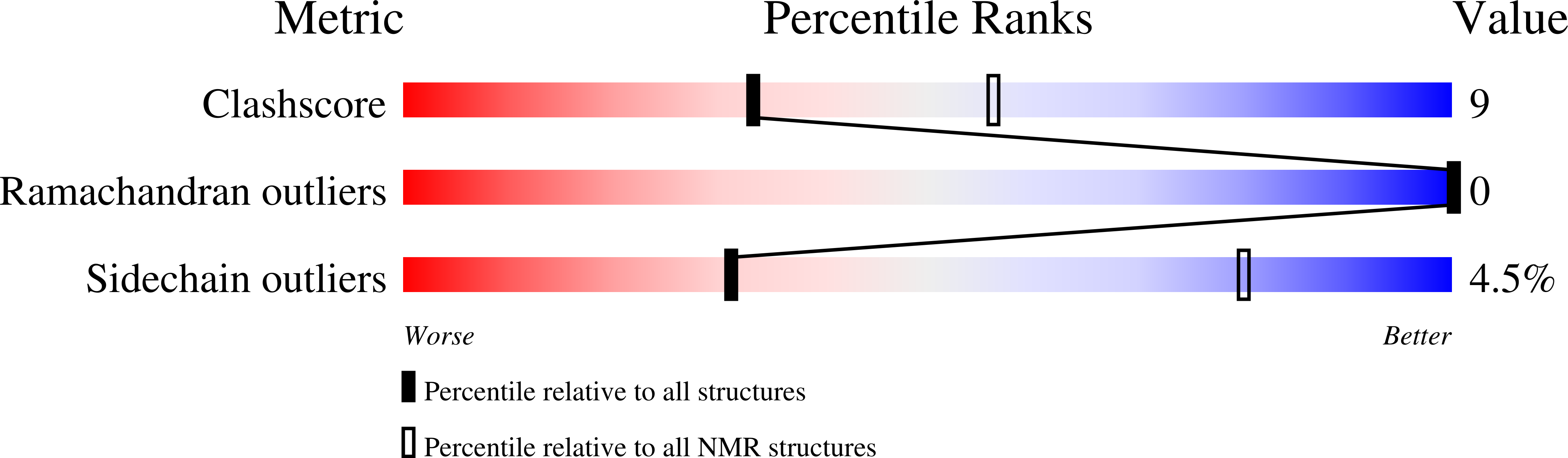
Deposition Date
2010-07-07
Release Date
2010-08-11
Last Version Date
2024-06-19
Entry Detail
PDB ID:
2XK0
Keywords:
Title:
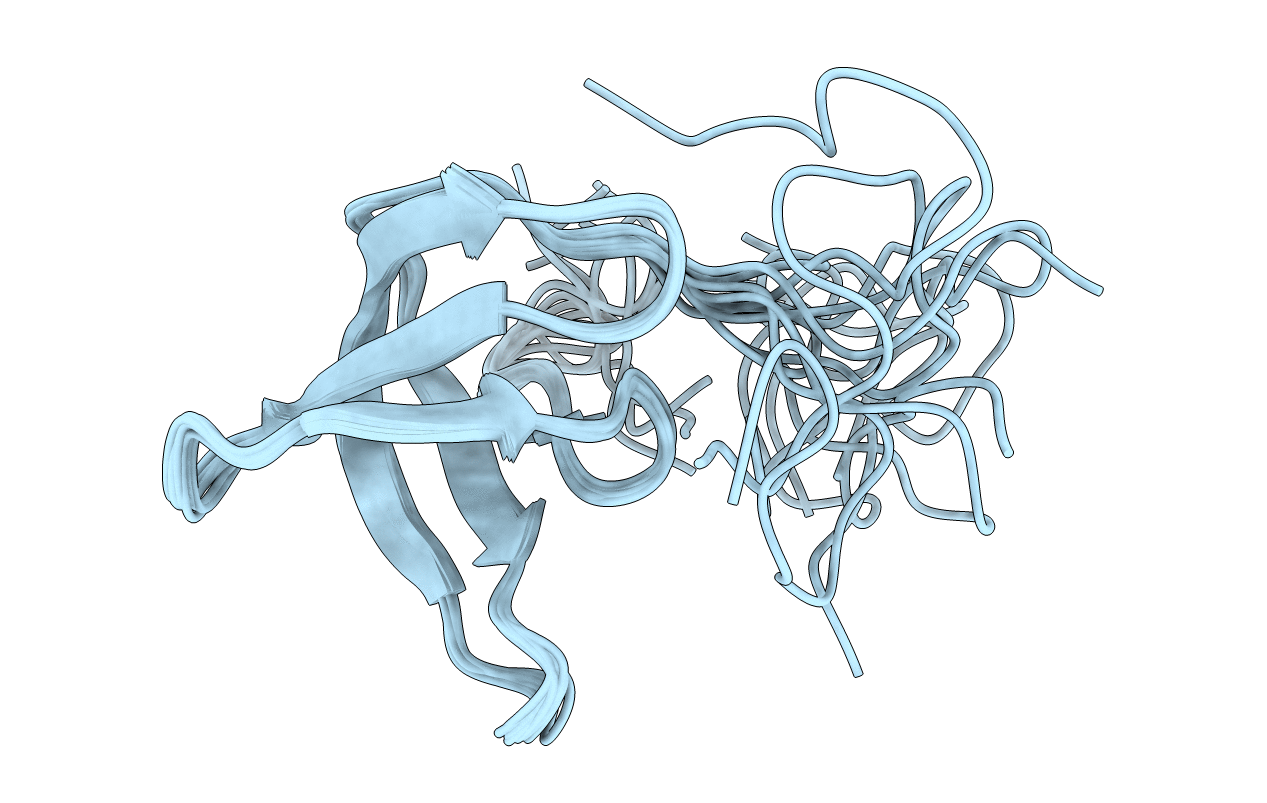
Solution structure of the Tudor domain from Drosophila Polycomblike (Pcl)
Biological Source:
Source Organism(s):
DROSOPHILA MELANOGASTER (Taxon ID: 7227)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
ENERGY