Deposition Date
2010-06-28
Release Date
2010-08-04
Last Version Date
2024-10-23
Entry Detail
PDB ID:
2XIC
Keywords:
Title:
Pilus-presented adhesin, Spy0125 (Cpa), P212121 form (ESRF data)
Biological Source:
Source Organism(s):
STREPTOCOCCUS PYOGENES (Taxon ID: 1314)
Expression System(s):
Method Details:
Experimental Method:
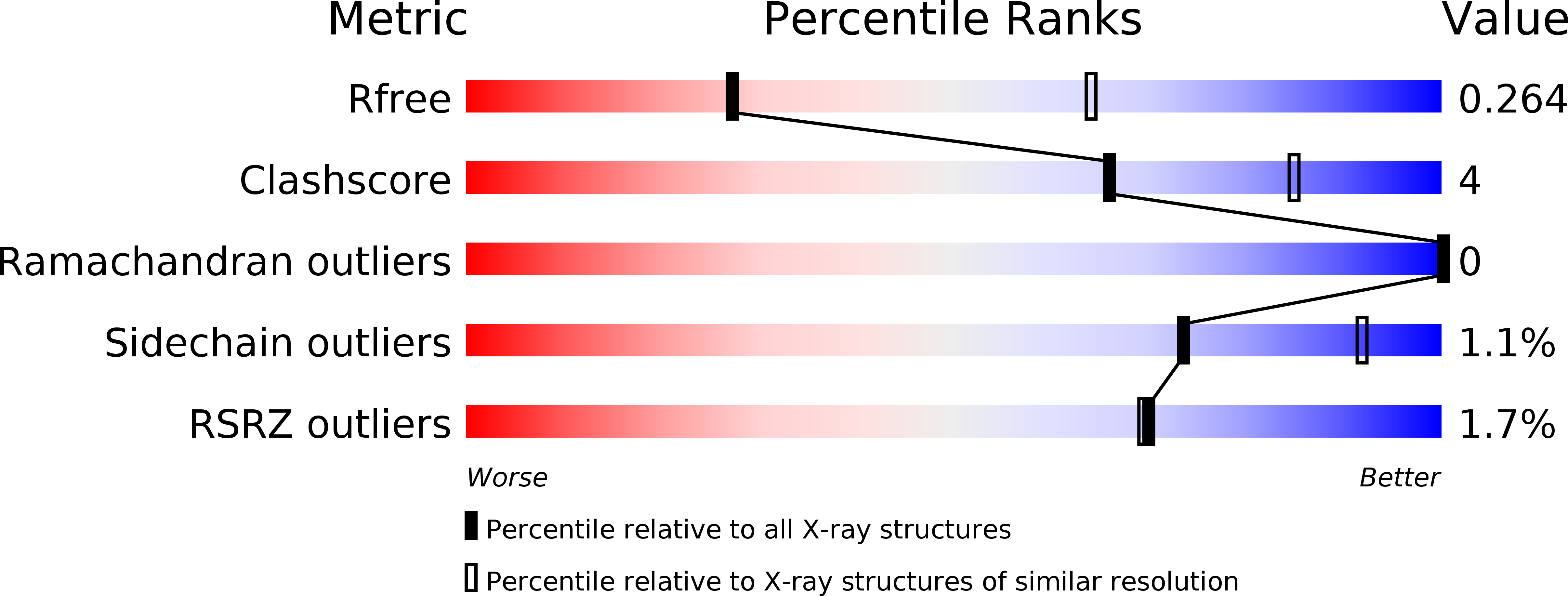
Resolution:
2.90 Å
R-Value Free:
0.27
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 21 21 21