Deposition Date
2010-01-25
Release Date
2011-01-19
Last Version Date
2023-12-20
Entry Detail
PDB ID:
2X3J
Keywords:
Title:
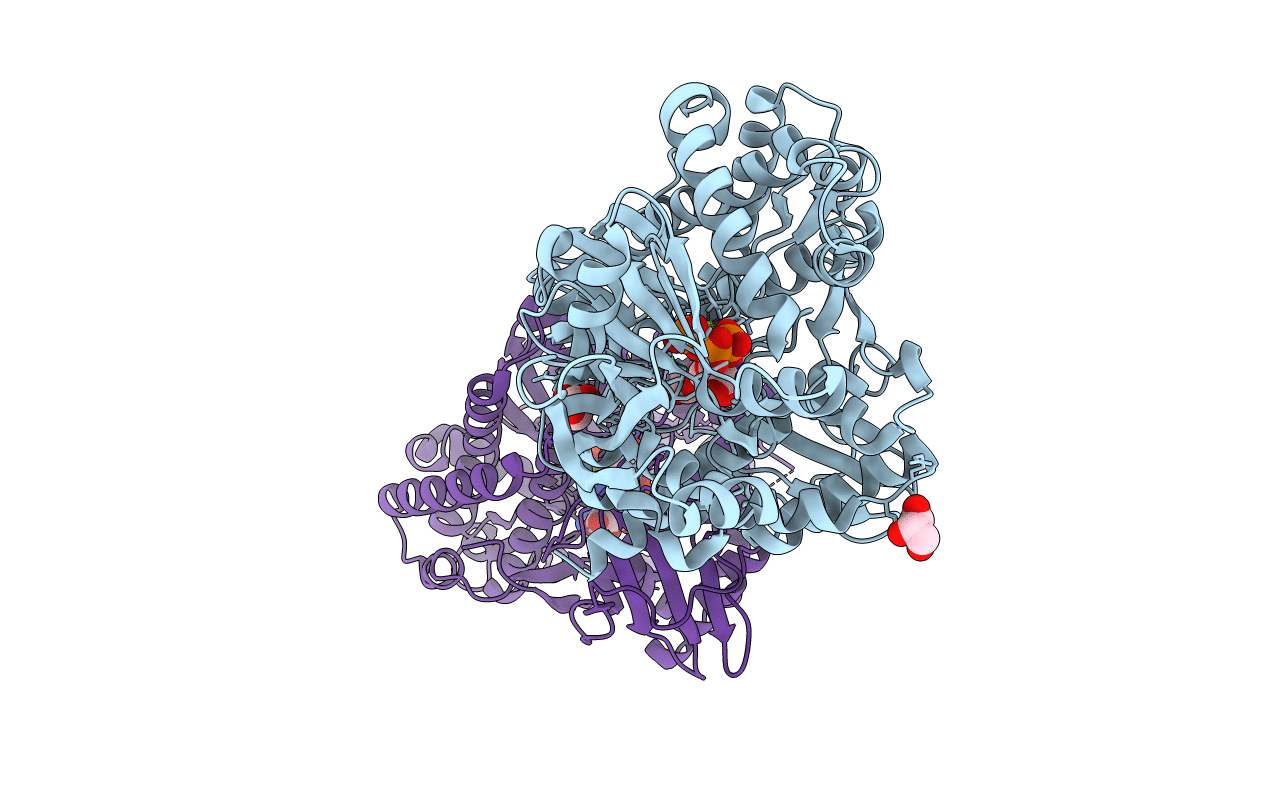
CO-COMPLEX STRUCTURE OF ACHROMOBACTIN SYNTHETASE PROTEIN D (ACSD) WITH ATP AND N-CITRYL-ETHYLENEDIAMINE FROM PECTOBACTERIUM CHRYSANTHEMI
Biological Source:
Source Organism(s):
ERWINIA CHRYSANTHEMI (Taxon ID: 556)
Expression System(s):
Method Details:
Experimental Method:
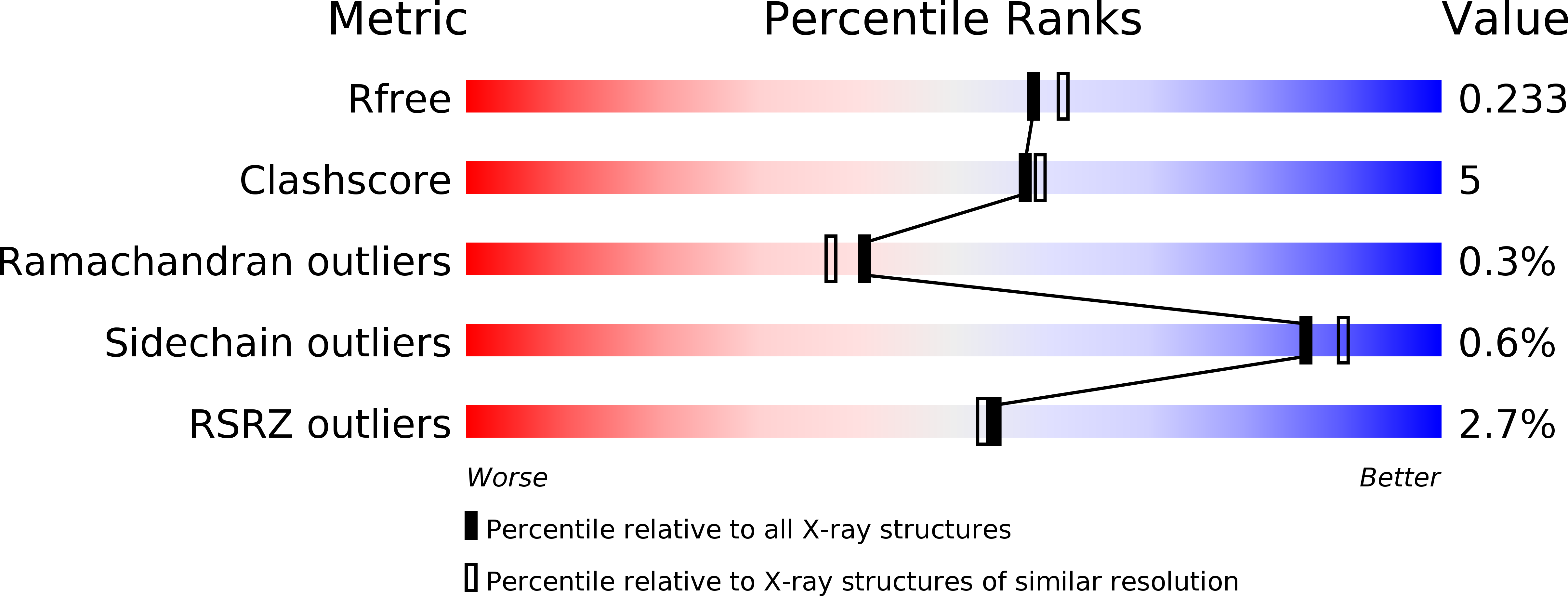
Resolution:
2.00 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
P 1