Deposition Date
2009-09-02
Release Date
2010-02-09
Last Version Date
2023-12-20
Entry Detail
PDB ID:
2WRV
Keywords:
Title:
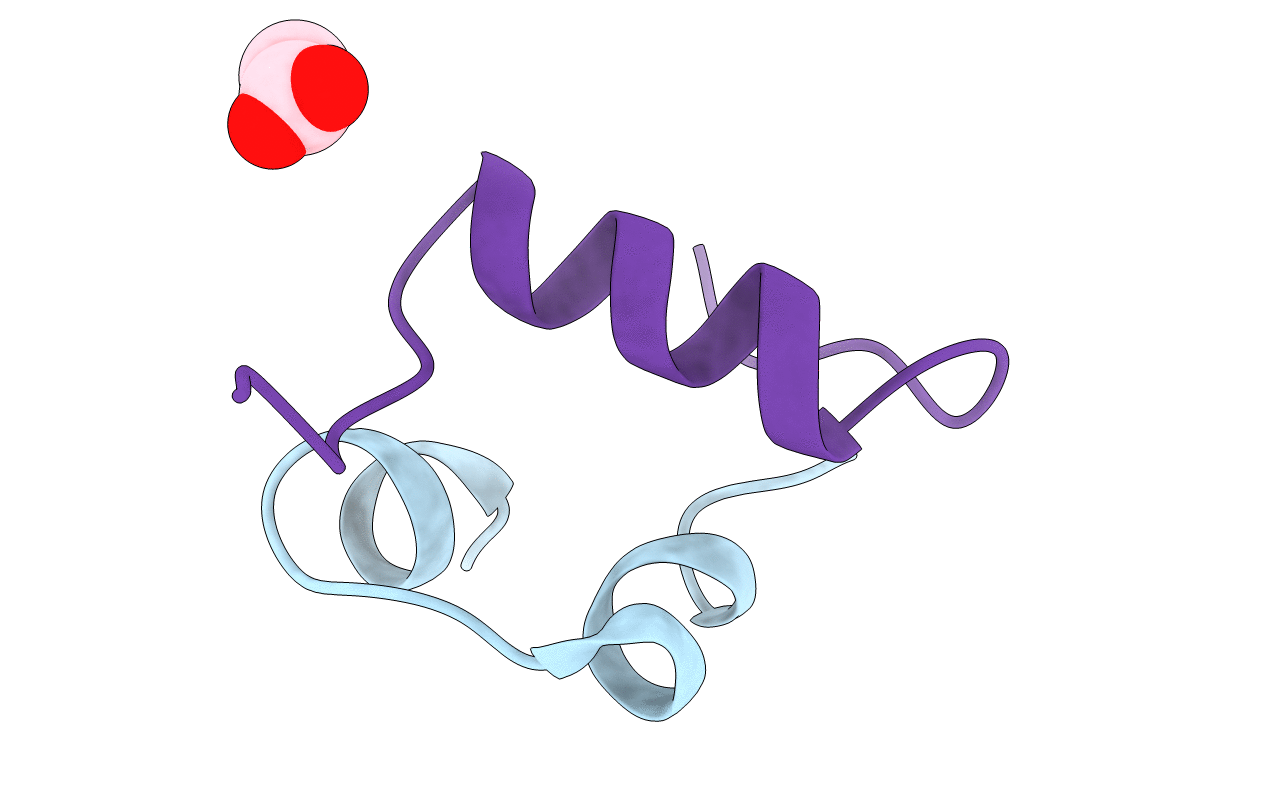
Semi-synthetic highly active analogue of human insulin NMeHisB26-DTI- NH2
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
Method Details:
Experimental Method:
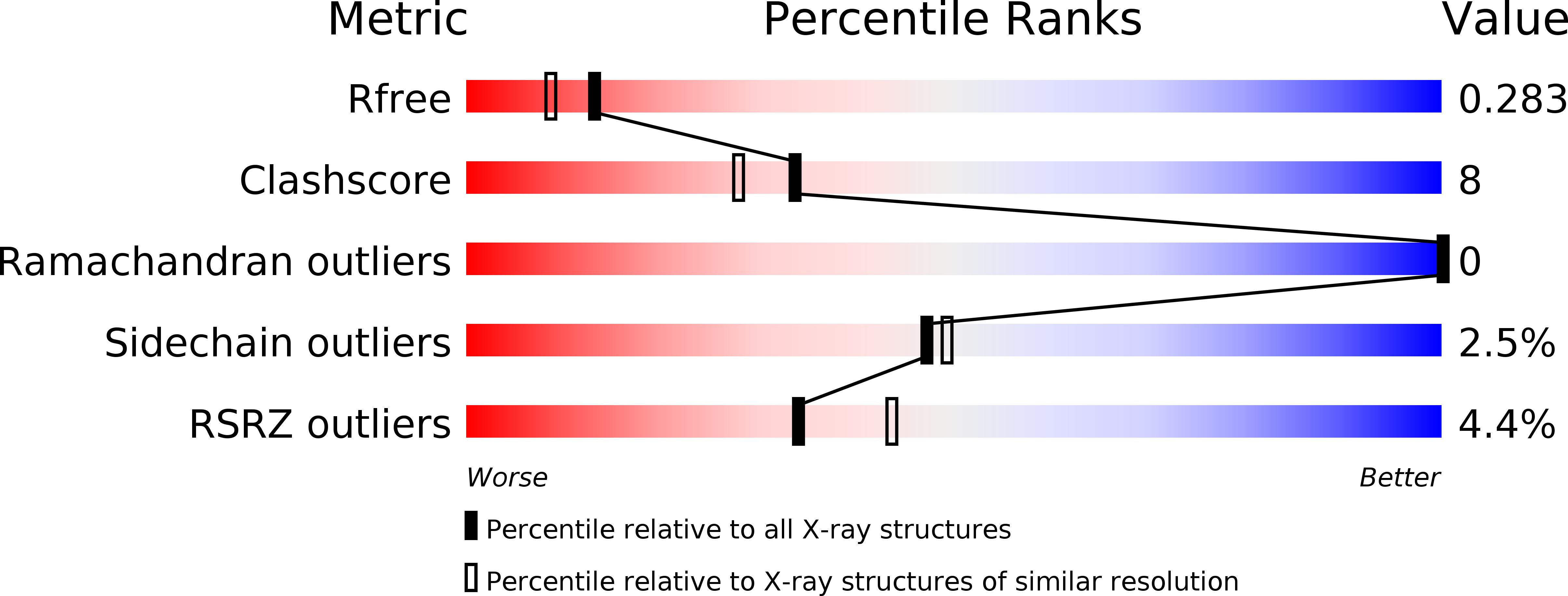
Resolution:
2.15 Å
R-Value Free:
0.27
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
I 41 2 2