Deposition Date
2009-08-09
Release Date
2009-11-03
Last Version Date
2023-12-20
Entry Detail
PDB ID:
2WPS
Keywords:
Title:
Salmonella enterica SadA 483-523 fused to GCN4 adaptors (SadAK3b-V2, out-of-register fusion)
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
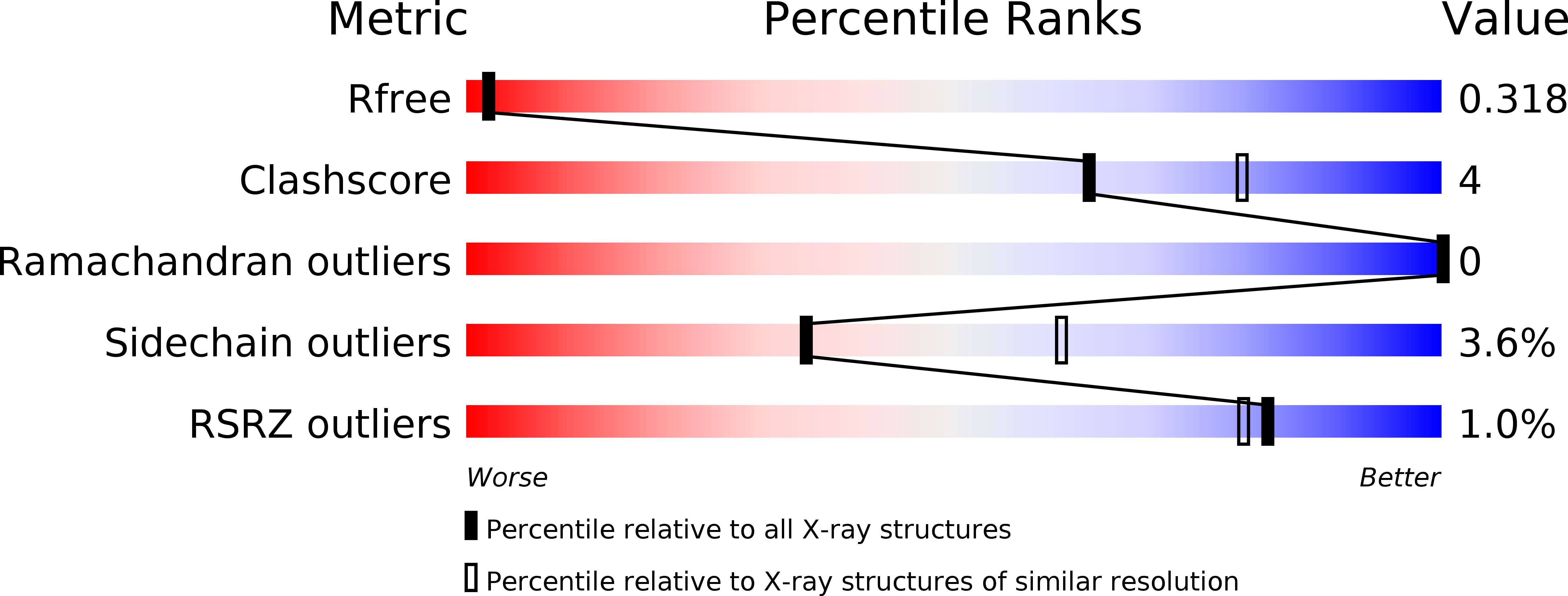
Resolution:
2.60 Å
R-Value Free:
0.32
R-Value Work:
0.23
R-Value Observed:
0.24
Space Group:
P 21 21 21