Deposition Date
2009-07-31
Release Date
2009-09-01
Last Version Date
2024-05-08
Entry Detail
PDB ID:
2WOZ
Keywords:
Title:
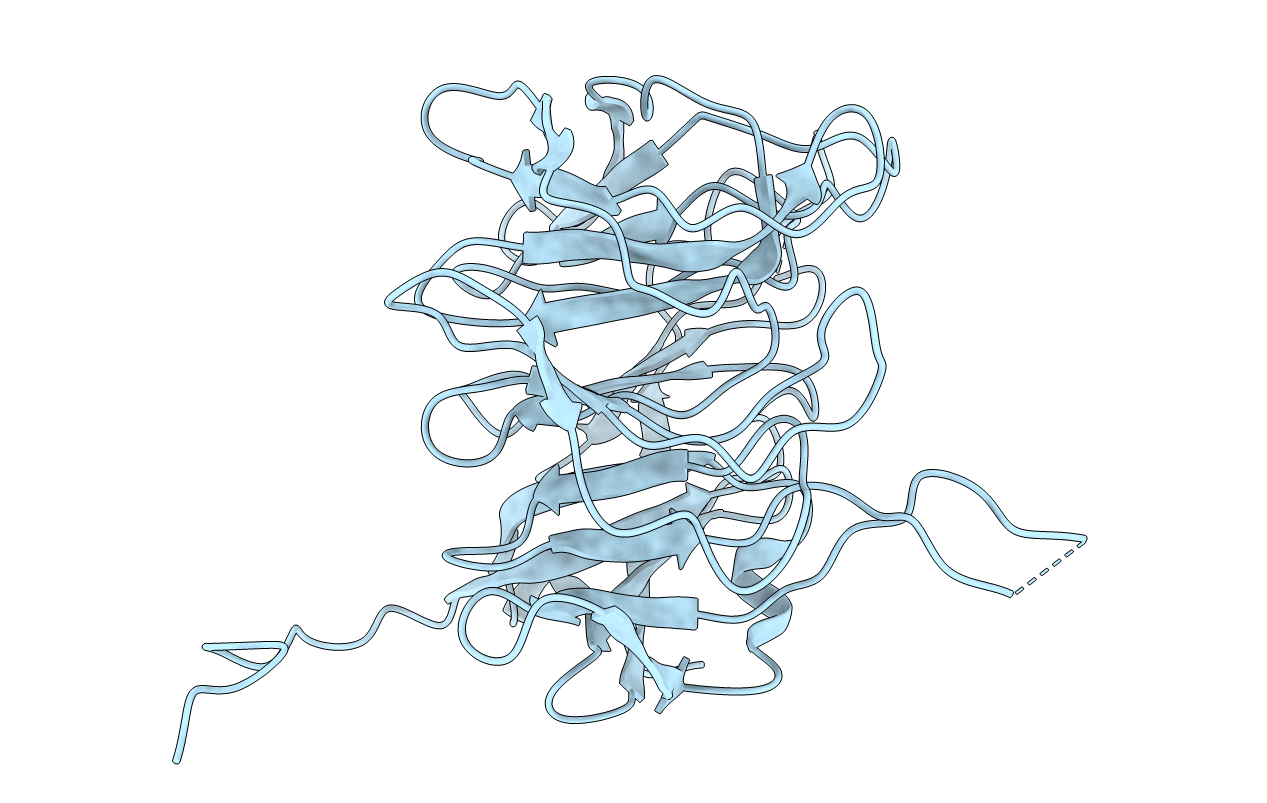
The novel beta-propeller of the BTB-Kelch protein Krp1 provides the binding site for Lasp-1 that is necessary for pseudopodia extension
Biological Source:
Source Organism(s):
RATTUS NORVEGICUS (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
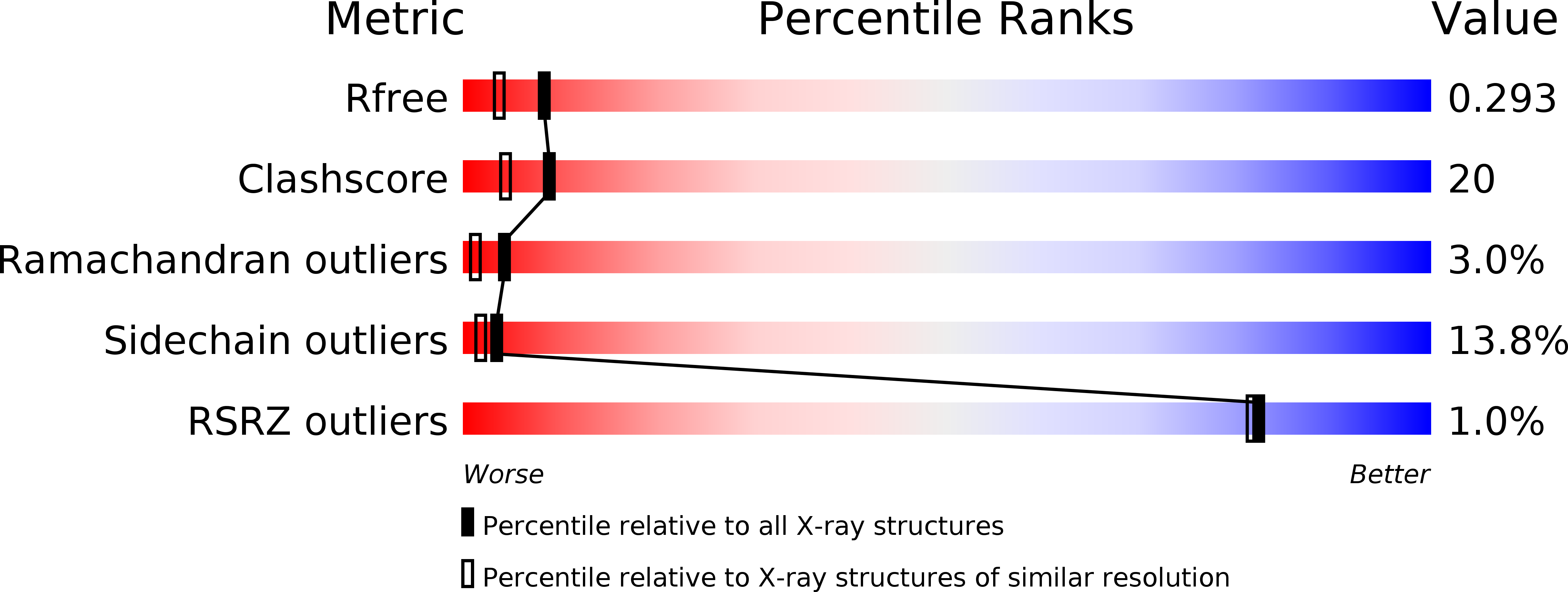
Resolution:
2.00 Å
R-Value Free:
0.27
R-Value Work:
0.23
Space Group:
P 21 21 2