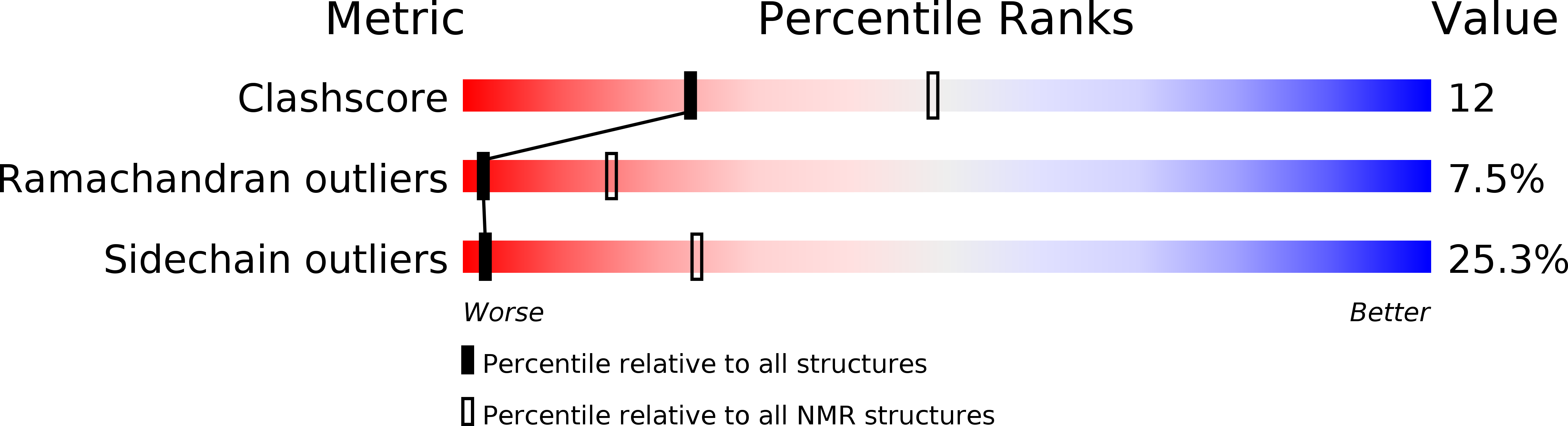
Deposition Date
2008-08-19
Release Date
2008-09-30
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2W0N
Keywords:
Title:
Plasticity of PAS domain and potential role for signal transduction in the histidine-kinase DcuS
Biological Source:
Source Organism(s):
ESCHERICHIA COLI (Taxon ID: 562)
Expression System(s):
Method Details:
Experimental Method:
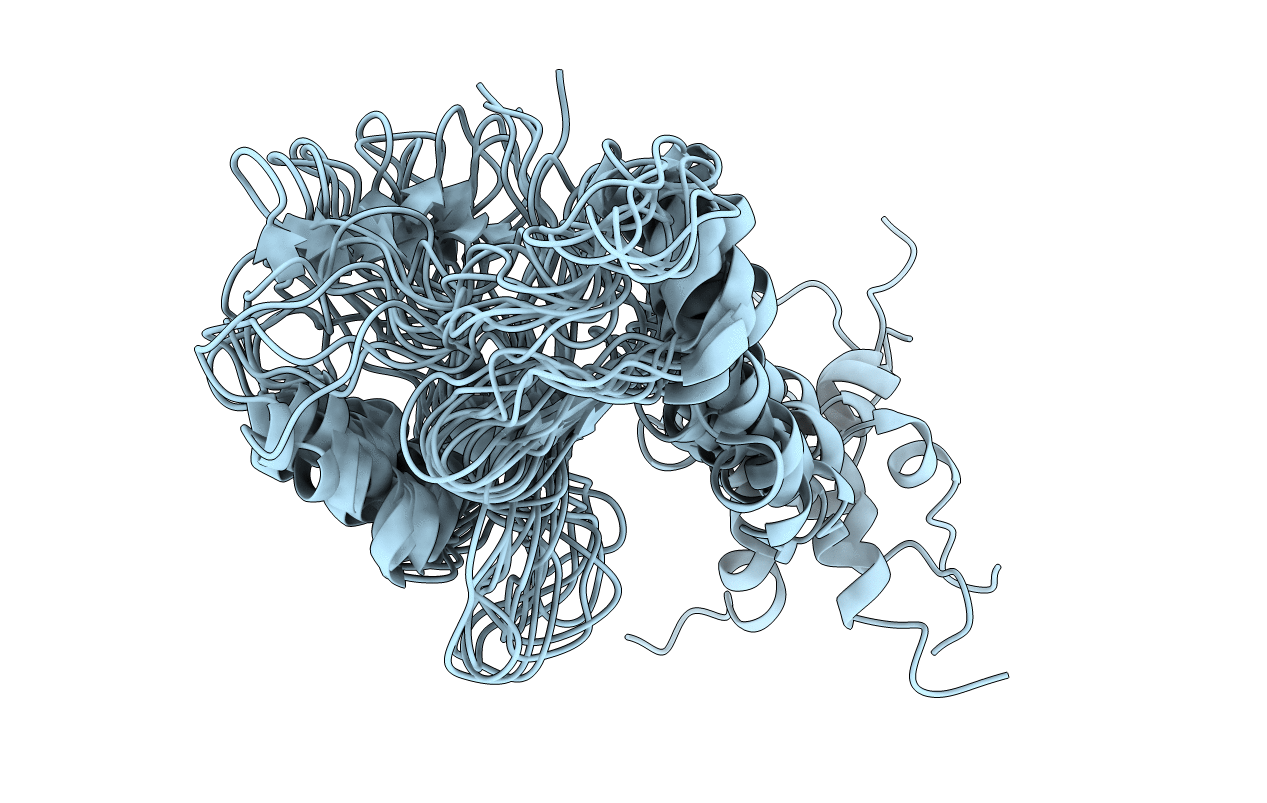
Conformers Submitted:
10