Deposition Date
2008-07-29
Release Date
2008-08-05
Last Version Date
2023-12-13
Entry Detail
PDB ID:
2VYV
Keywords:
Title:
Structure of E.Coli GAPDH Rat Sperm GAPDH heterotetramer
Biological Source:
Source Organism:
ESCHERICHIA COLI BL21(DE3) (Taxon ID: 469008)
RATTUS NORVEGICUS (Taxon ID: 10116)
RATTUS NORVEGICUS (Taxon ID: 10116)
Method Details:
Experimental Method:
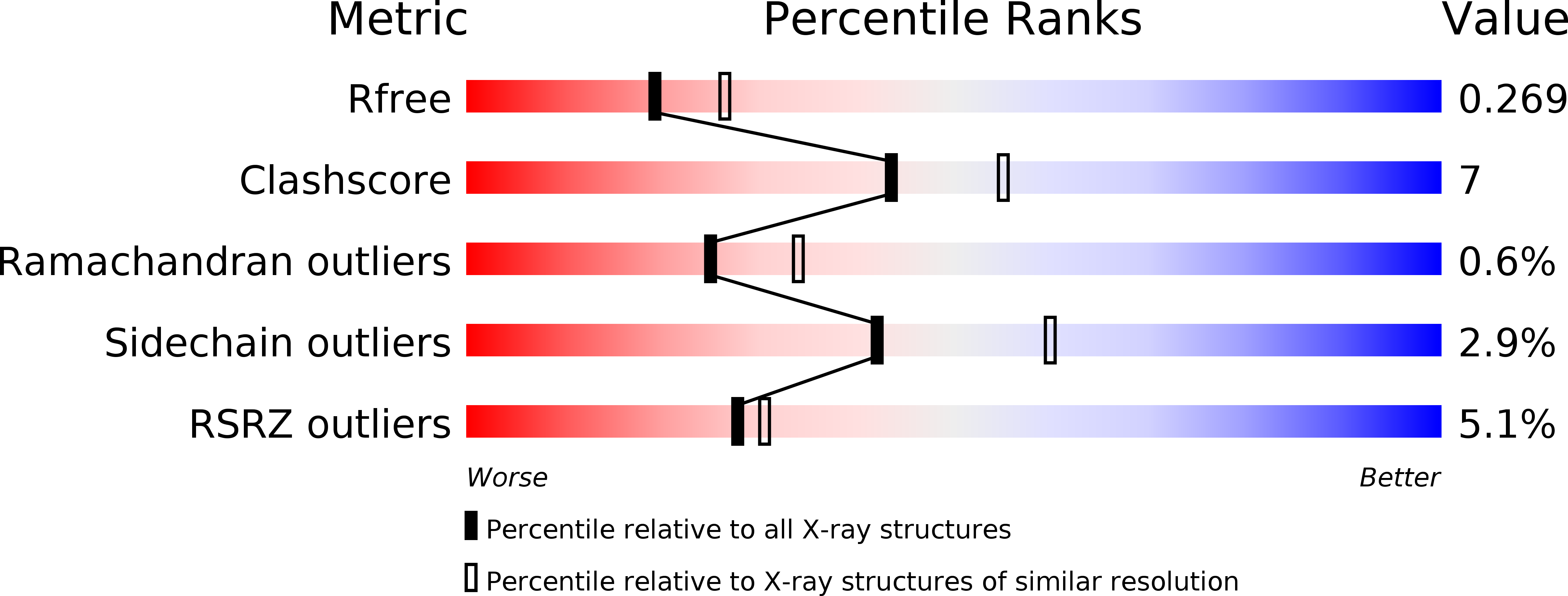
Resolution:
2.38 Å
R-Value Free:
0.25
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 21