Deposition Date
2008-05-14
Release Date
2008-09-09
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2VTC
Keywords:
Title:
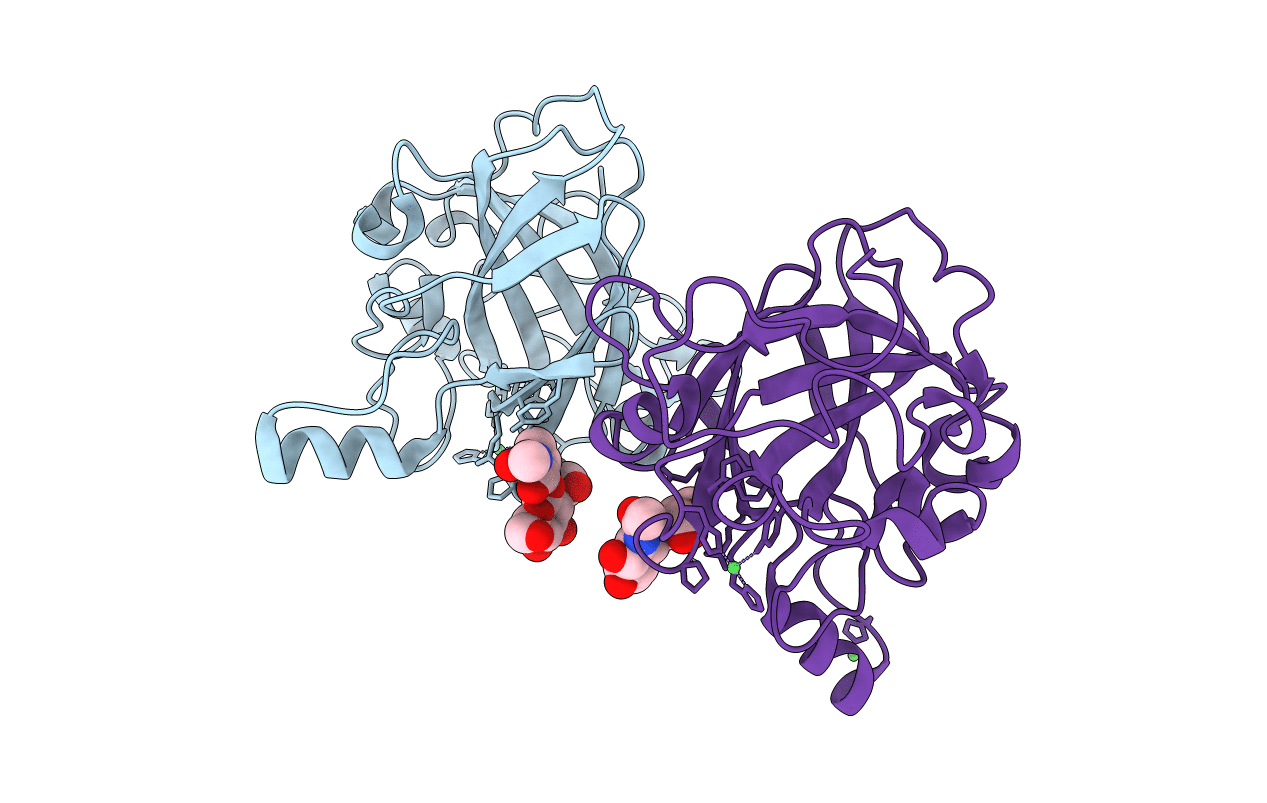
The structure of a glycoside hydrolase family 61 member, Cel61B from the Hypocrea jecorina.
Biological Source:
Source Organism(s):
HYPOCREA JECORINA (Taxon ID: 51453)
Method Details:
Experimental Method:
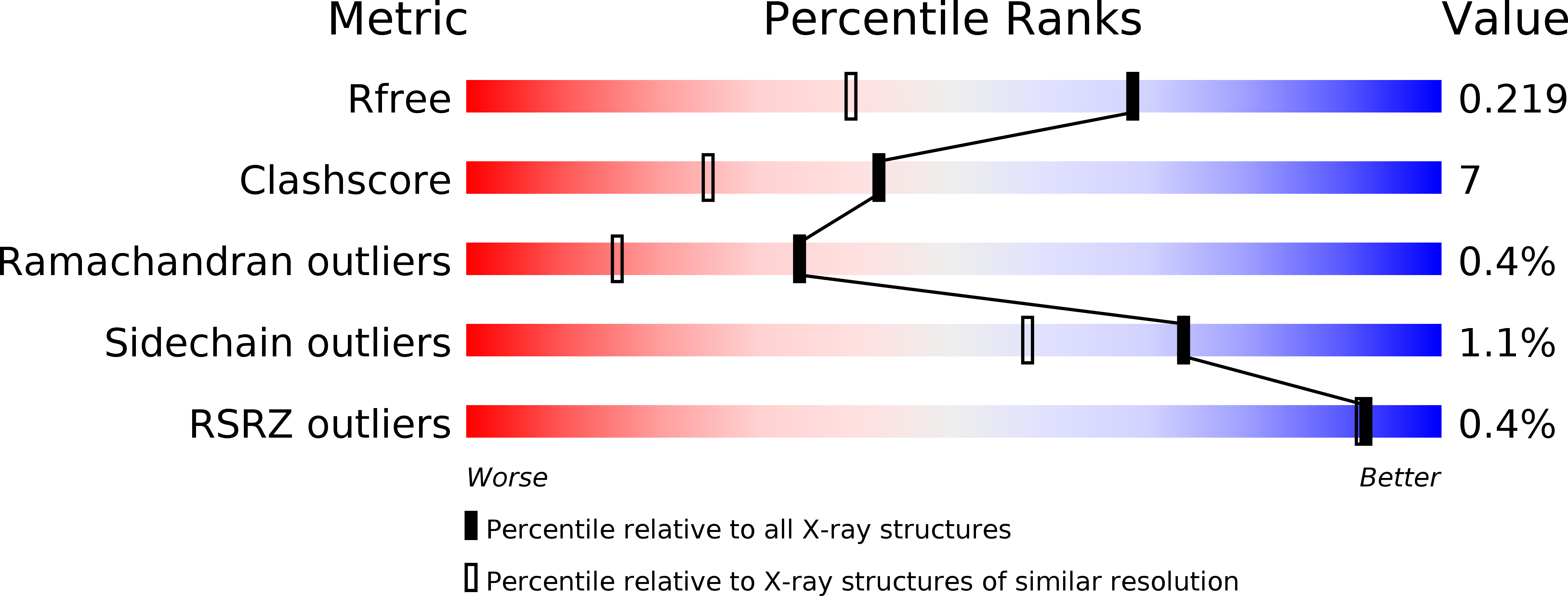
Resolution:
1.60 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 65