Deposition Date
2008-04-16
Release Date
2008-06-17
Last Version Date
2024-05-08
Entry Detail
PDB ID:
2VRW
Keywords:
Title:
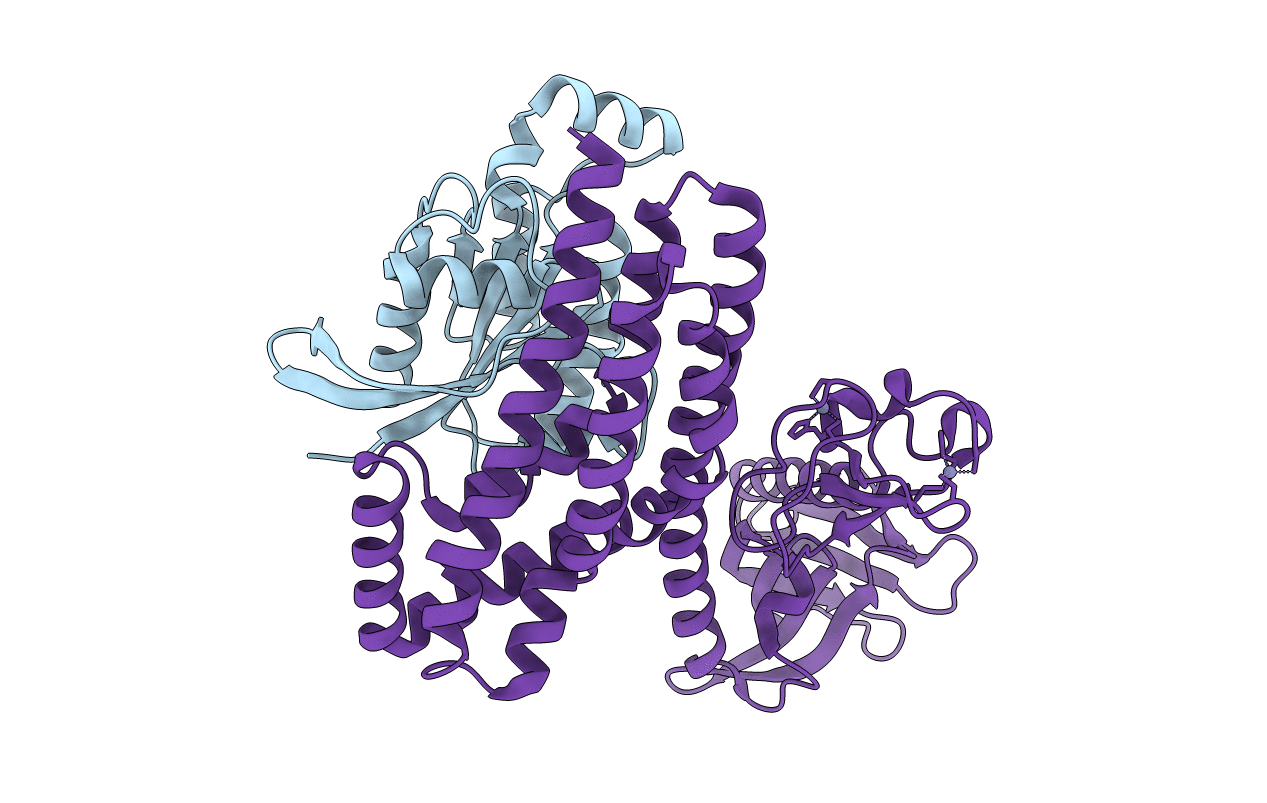
Critical structural role for the PH and C1 domains of the Vav1 exchange factor
Biological Source:
Source Organism:
HOMO SAPIENS (Taxon ID: 9606)
MUS MUSCULUS (Taxon ID: 10090)
MUS MUSCULUS (Taxon ID: 10090)
Host Organism:
Method Details:
Experimental Method:
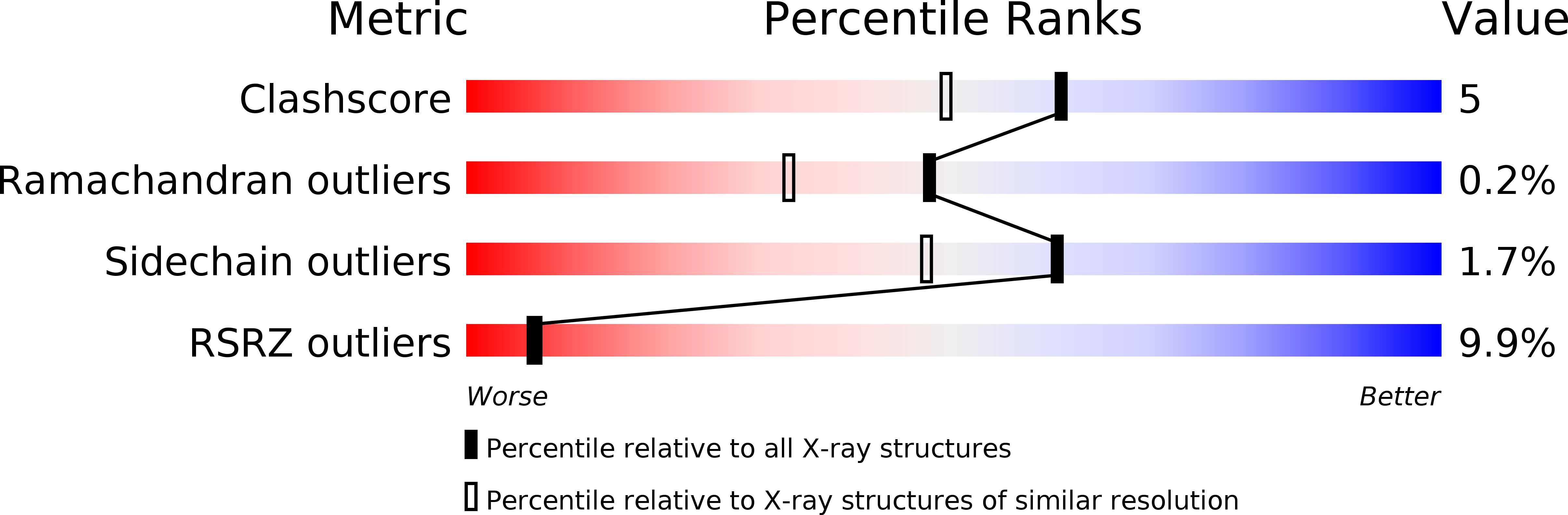
Resolution:
1.85 Å
R-Value Free:
0.25
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
C 1 2 1