Deposition Date
2008-02-13
Release Date
2009-03-10
Last Version Date
2024-10-23
Entry Detail
PDB ID:
2VOC
Keywords:
Title:
THIOREDOXIN A ACTIVE SITE MUTANTS FORM MIXED DISULFIDE DIMERS THAT RESEMBLE ENZYME-SUBSTRATE REACTION INTERMEDIATE
Biological Source:
Source Organism(s):
Bacillus subtilis subsp. subtilis str. 168 (Taxon ID: 224308)
Expression System(s):
Method Details:
Experimental Method:
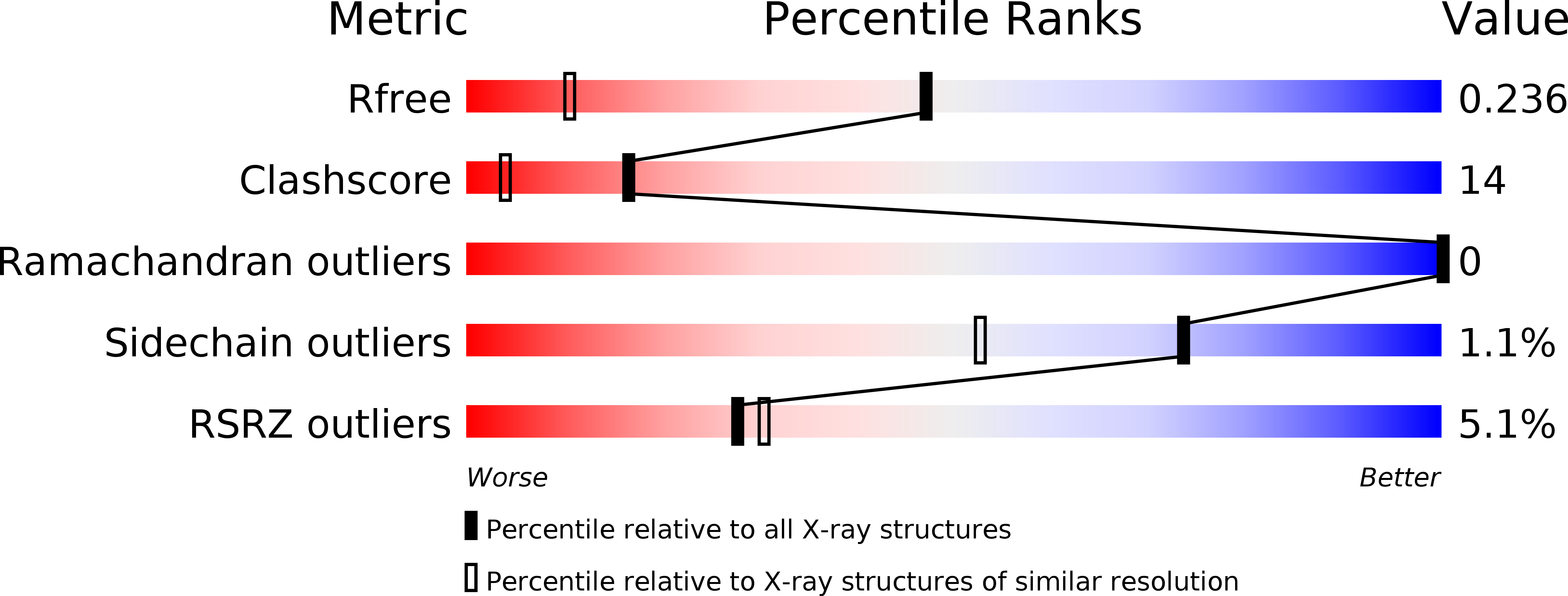
Resolution:
1.50 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 1