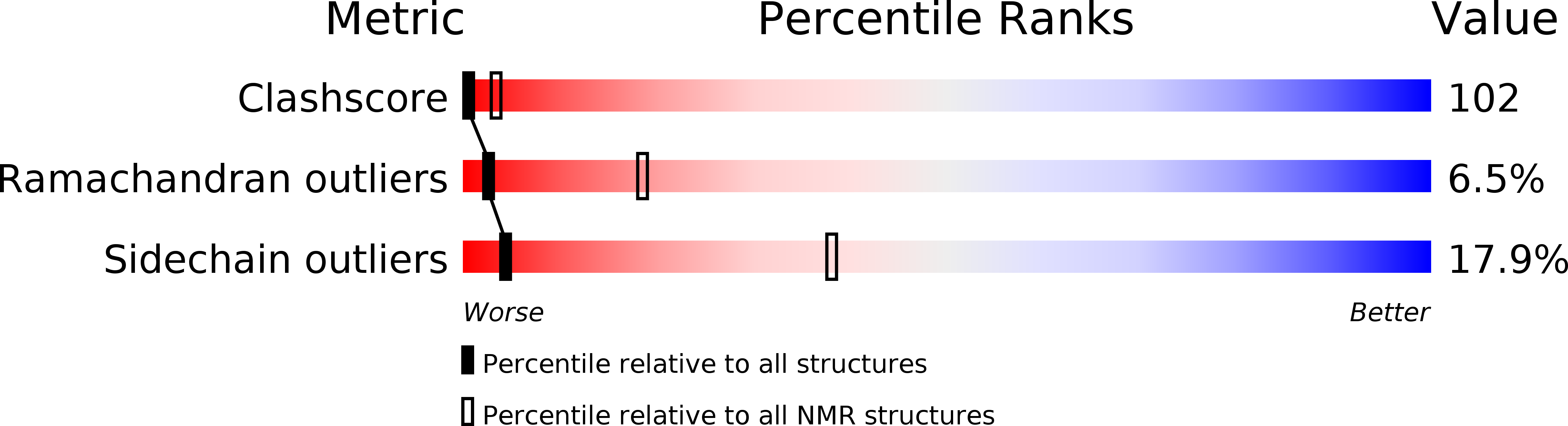
Deposition Date
1997-01-16
Release Date
1997-04-01
Last Version Date
2024-05-01
Entry Detail
PDB ID:
2VIK
Keywords:
Title:
REFINED STRUCTURE OF THE ACTIN-SEVERING DOMAIN VILLIN 14T, DETERMINED BY SOLUTION NMR, MINIMIZED AVERAGE STRUCTURE
Biological Source:
Source Organism(s):
Gallus gallus (Taxon ID: 9031)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
1
Selection Criteria:
NOE VIOLATIONS < 0.5 A DIHEDRAL VIOL < 5 DEGREES