Deposition Date
2007-10-24
Release Date
2008-02-19
Last Version Date
2023-12-13
Entry Detail
PDB ID:
2VEI
Keywords:
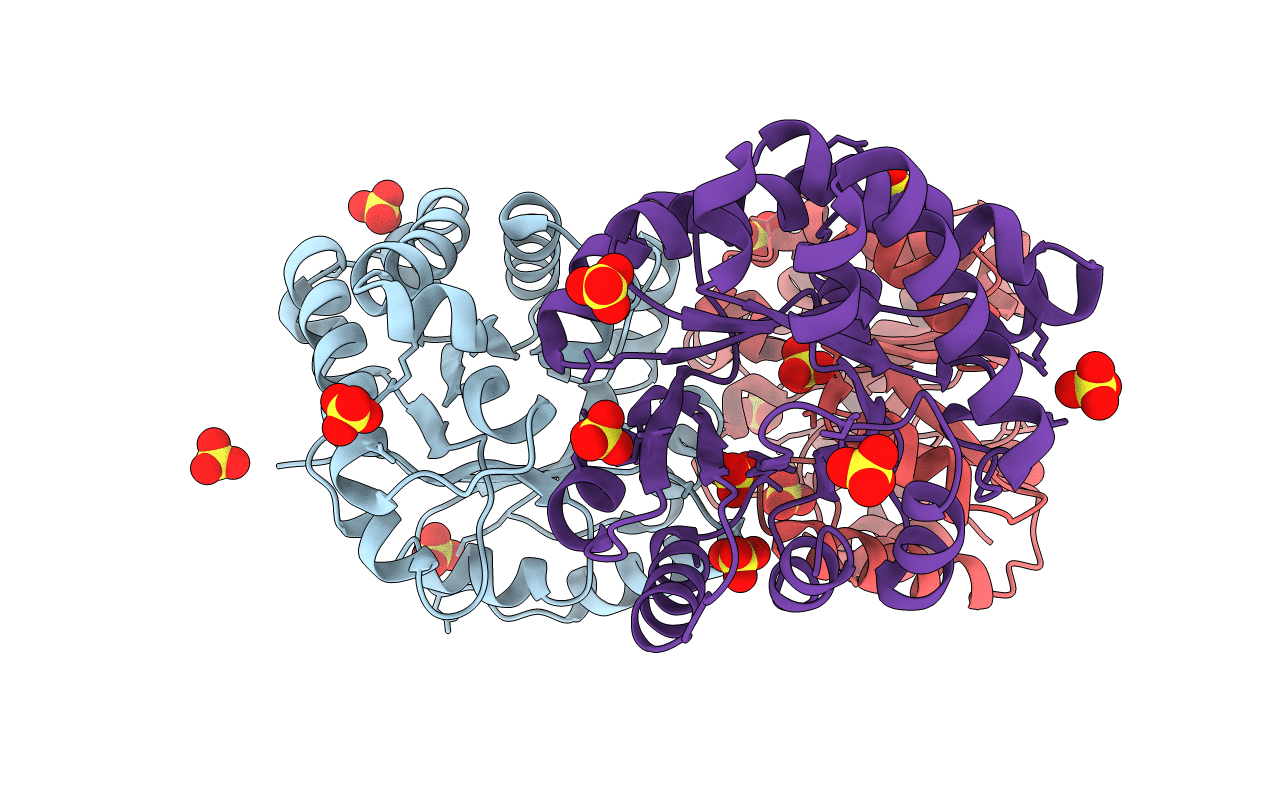
Title:
Structure-based enzyme engineering efforts with an inactive monomeric TIM variant: the importance of a single point mutation for generating an active site with suitable binding properties
Biological Source:
Source Organism(s):
TRYPANOSOMA BRUCEI BRUCEI (Taxon ID: 5702)
Expression System(s):
Method Details:
Experimental Method:
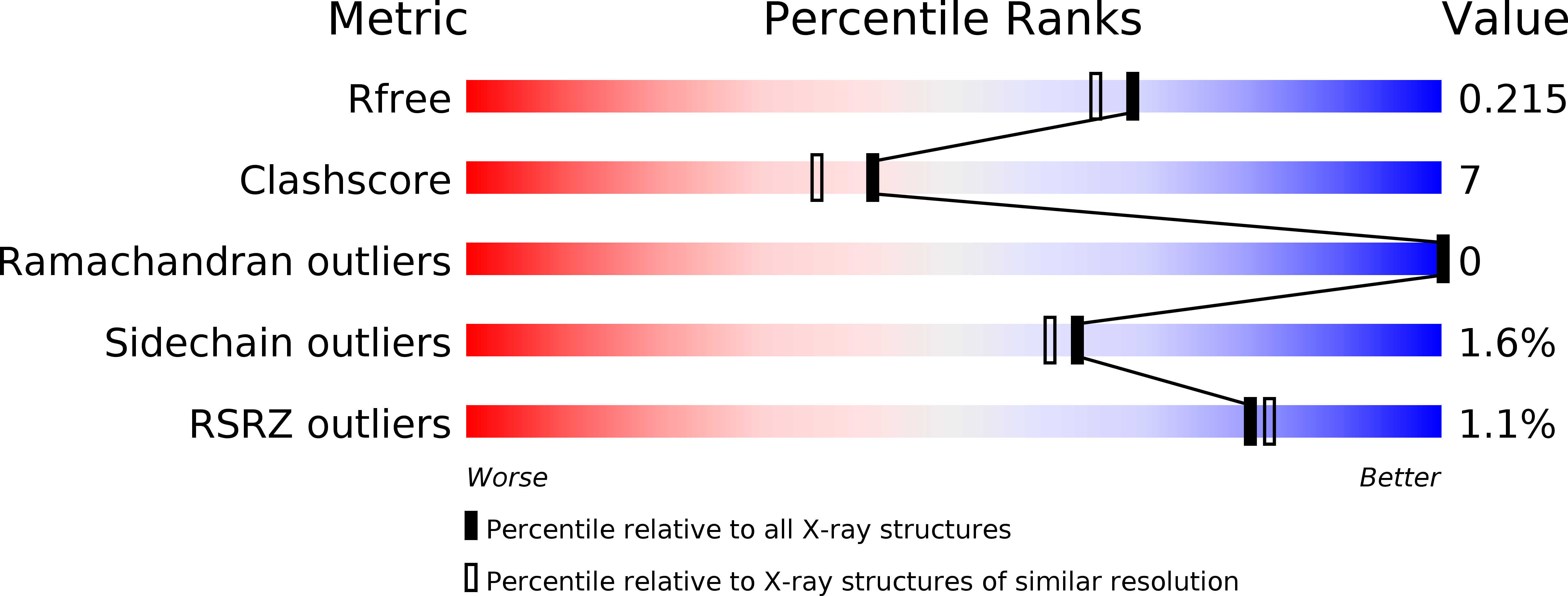
Resolution:
1.89 Å
R-Value Free:
0.21
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 1 21 1