Deposition Date
2007-10-04
Release Date
2008-01-15
Last Version Date
2024-05-08
Entry Detail
PDB ID:
2VDC
Keywords:
Title:
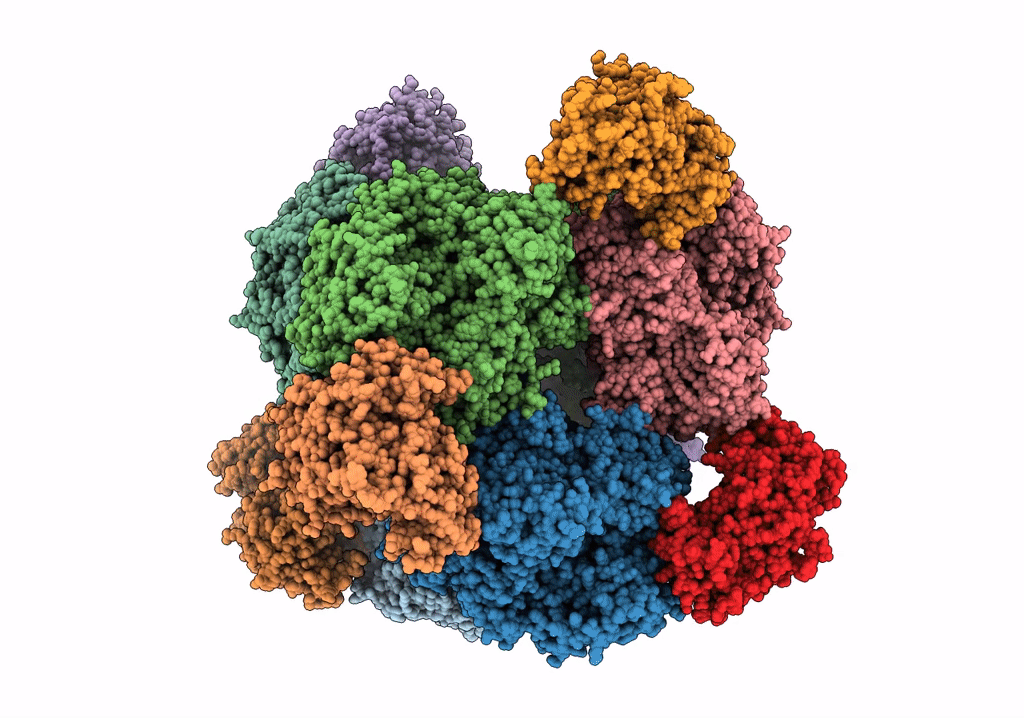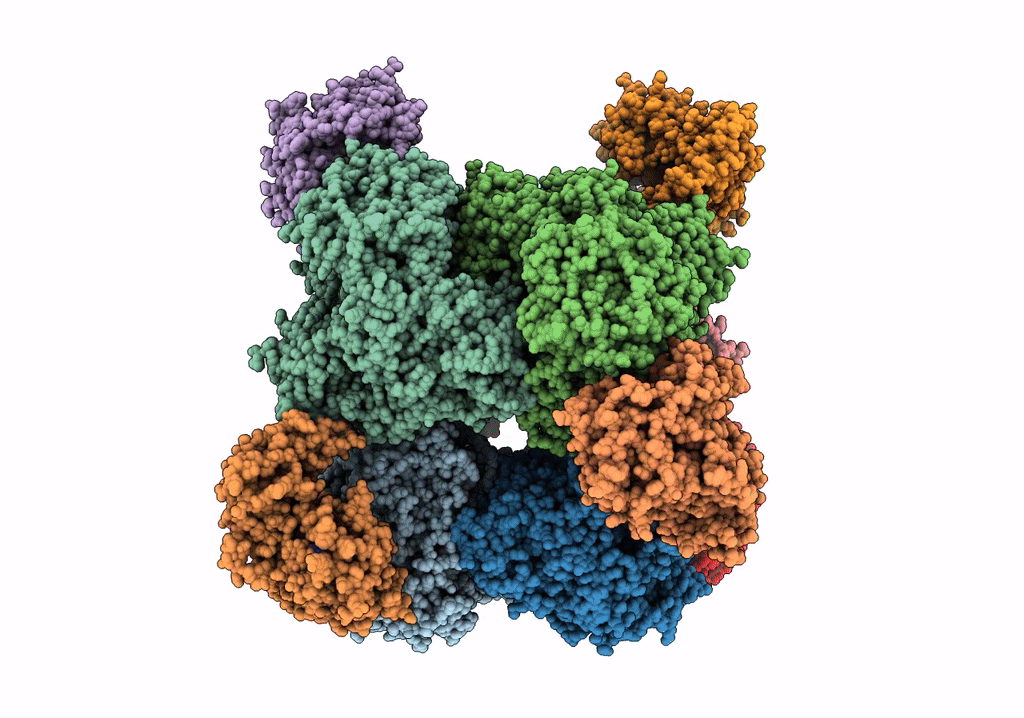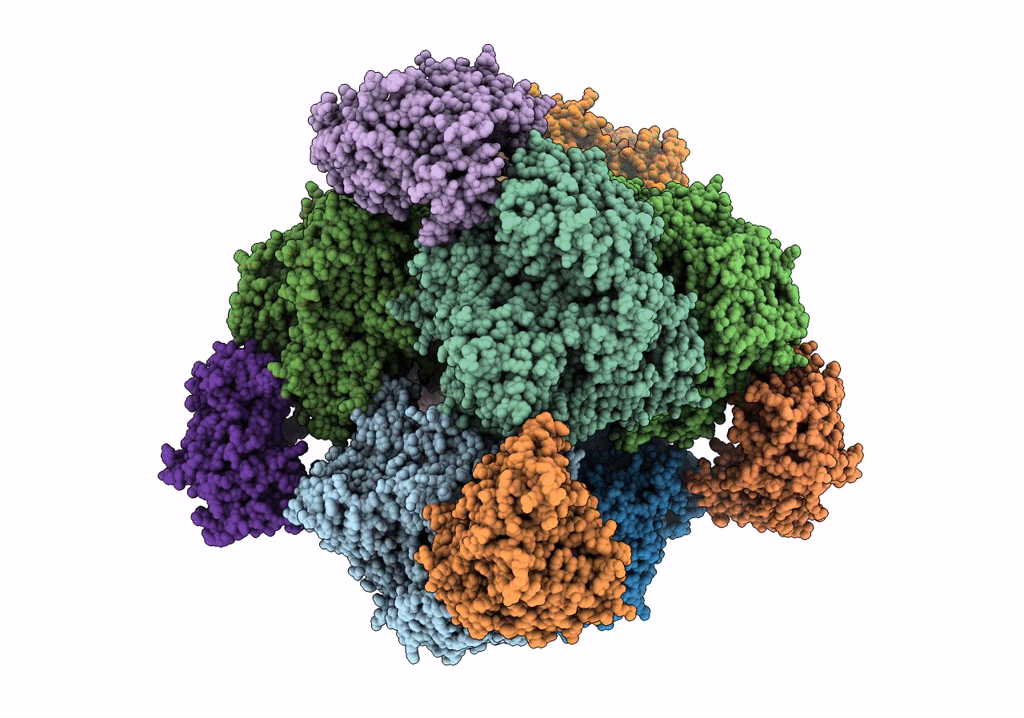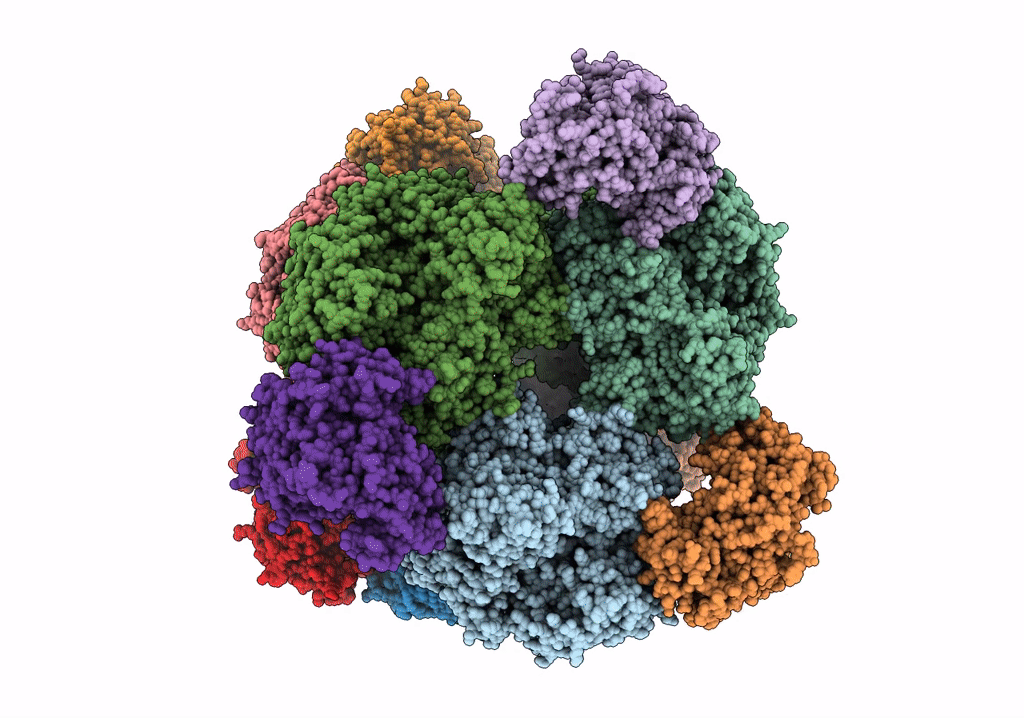
THE 9.5 A RESOLUTION STRUCTURE OF GLUTAMATE SYNTHASE FROM CRYO-ELECTRON MICROSCOPY AND ITS OLIGOMERIZATION BEHAVIOR IN SOLUTION: FUNCTIONAL IMPLICATIONS.
Biological Source:
Source Organism(s):
AZOSPIRILLUM BRASILENSE (Taxon ID: 192)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
9.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


