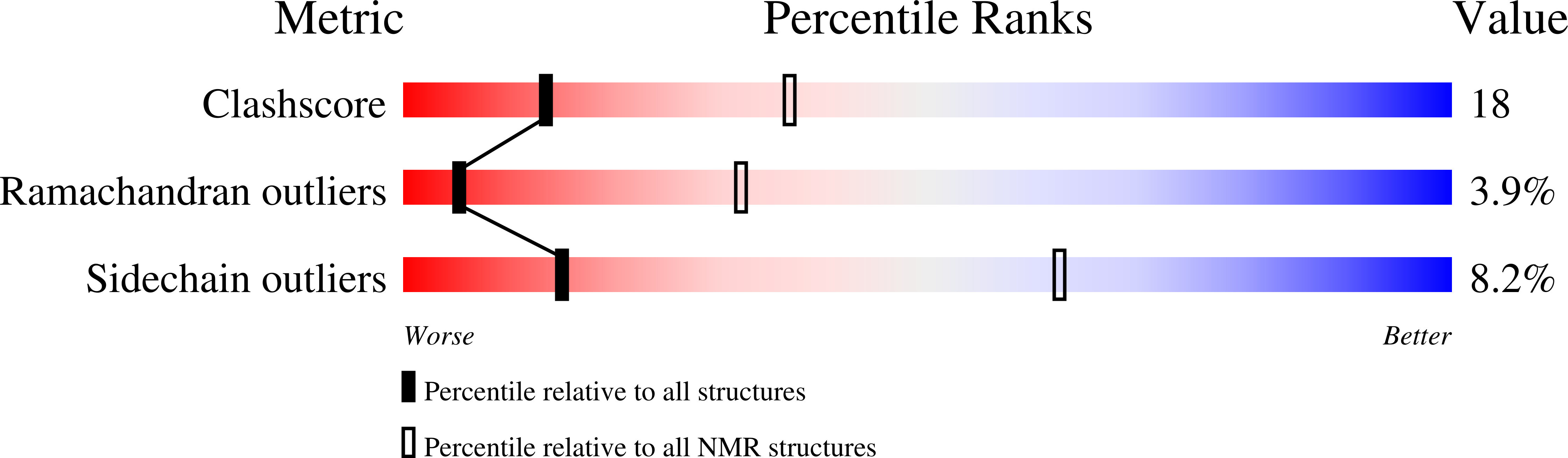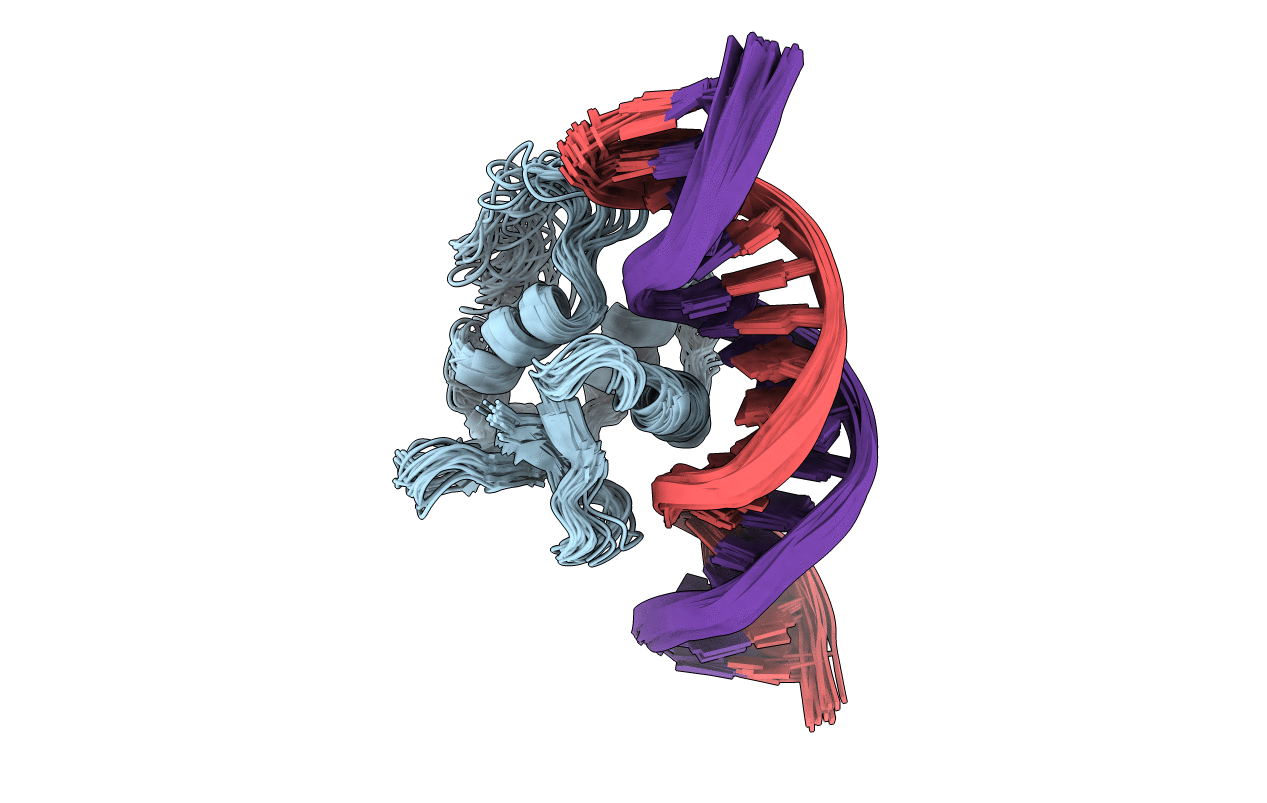
Deposition Date
1996-08-05
Release Date
1997-03-12
Last Version Date
2024-05-22
Entry Detail
PDB ID:
2STT
Keywords:
Title:
SOLUTION NMR STRUCTURE OF THE HUMAN ETS1/DNA COMPLEX, 25 STRUCTURES
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
Conformers Submitted:
25