Deposition Date
1998-10-03
Release Date
1999-04-27
Last Version Date
2024-04-03
Entry Detail
PDB ID:
2SLI
Keywords:
Title:
LEECH INTRAMOLECULAR TRANS-SIALIDASE COMPLEXED WITH 2,7-ANHYDRO-NEU5AC, THE REACTION PRODUCT
Biological Source:
Source Organism(s):
Macrobdella decora (Taxon ID: 6405)
Expression System(s):
Method Details:
Experimental Method:
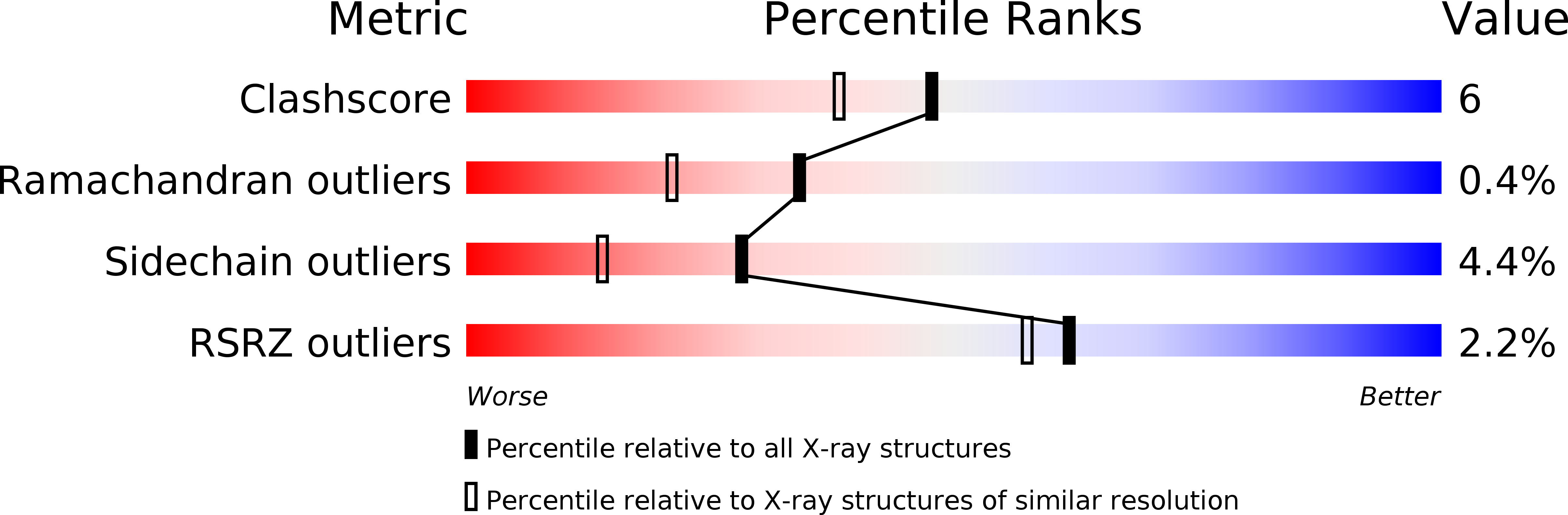
Resolution:
1.80 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 1