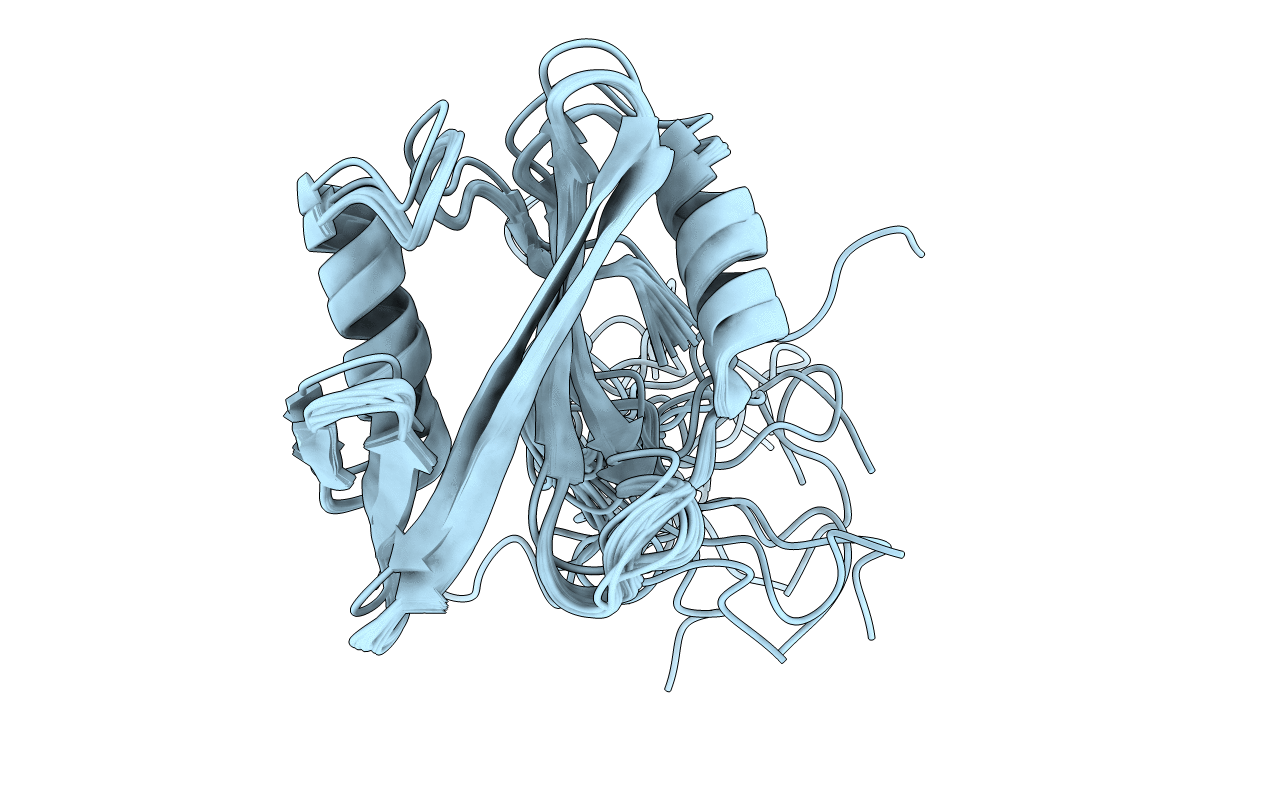
Deposition Date
2015-09-24
Release Date
2016-09-21
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2RVF
Keywords:
Title:
Solution NMR structure of Monosiga brevicollis CRK/CRKL homolog (crka1) SH2 domain
Biological Source:
Source Organism(s):
Monosiga brevicollis (Taxon ID: 81824)
Expression System(s):
Method Details:
Experimental Method:
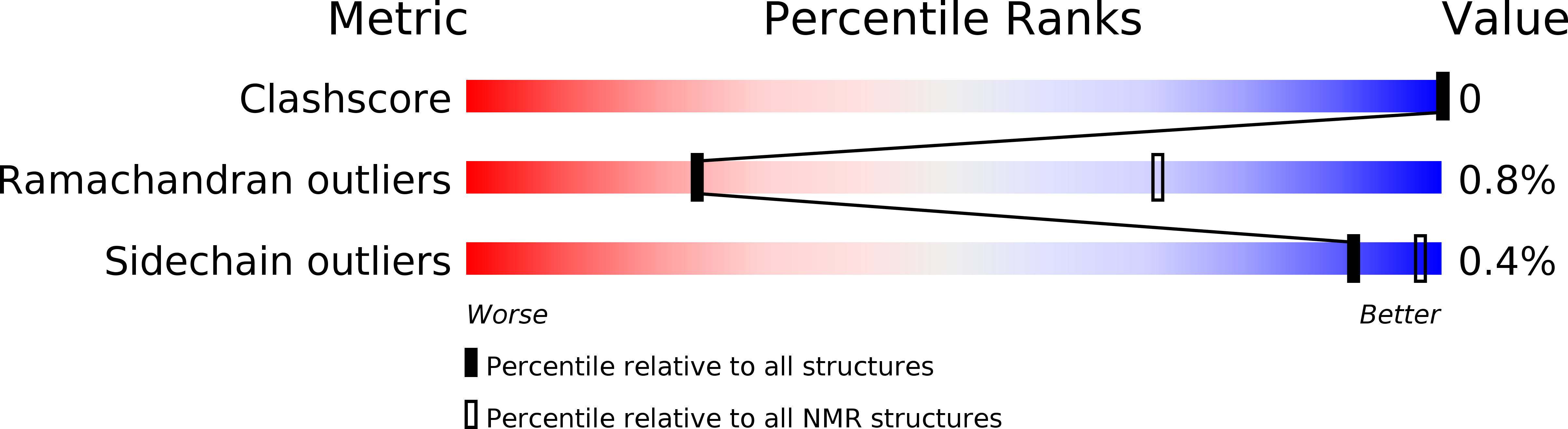
Conformers Calculated:
100
Conformers Submitted:
21
Selection Criteria:
target function