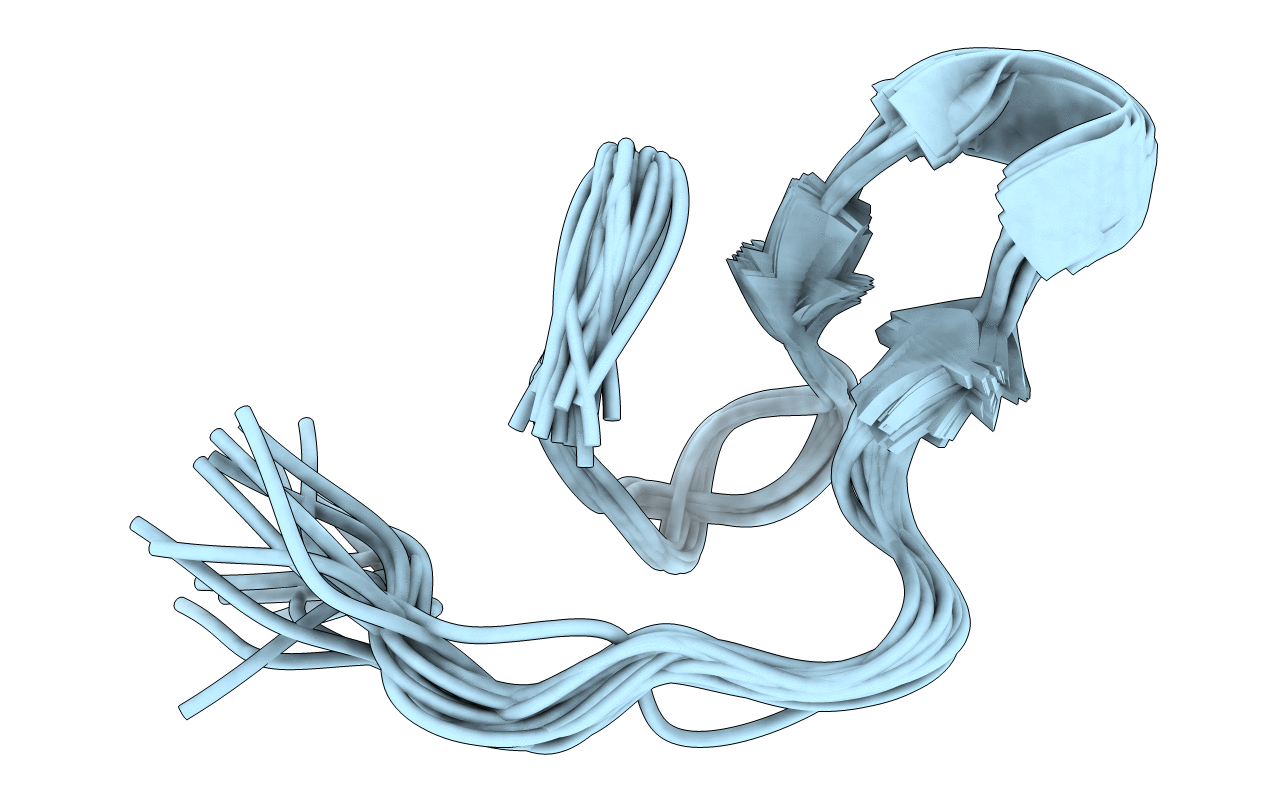
Deposition Date
2013-04-18
Release Date
2014-01-01
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2RT4
Keywords:
Title:
NMR Structure of designed protein, AF.2A1, (Ensembles)
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy