Deposition Date
2012-04-18
Release Date
2012-08-29
Last Version Date
2025-03-26
Entry Detail
PDB ID:
2RSN
Keywords:
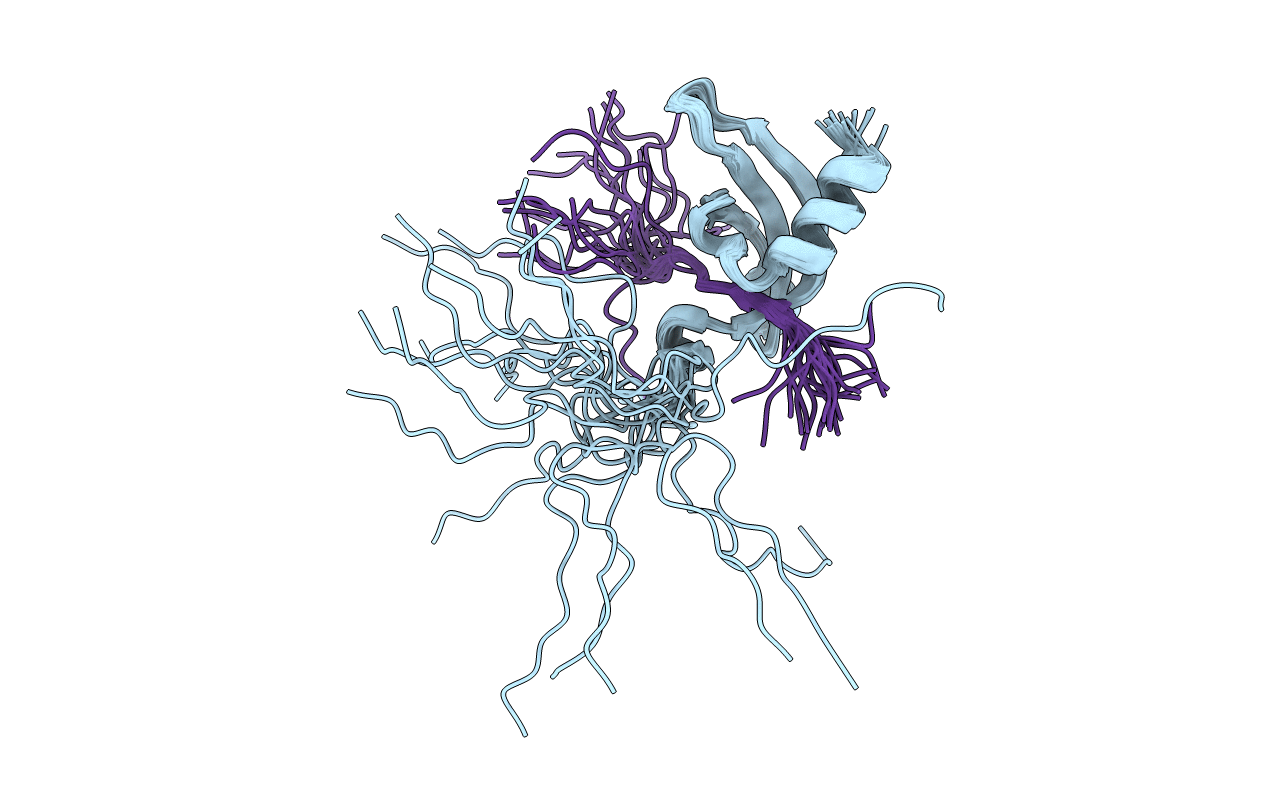
Title:
Solution structure of the chromodomain of Chp1 in complex with H3K9me3 peptide
Biological Source:
Source Organism(s):
Schizosaccharomyces pombe (Taxon ID: 284812)
Schizosaccharomyces pombe (Taxon ID: 4896)
Schizosaccharomyces pombe (Taxon ID: 4896)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy


