Abstact
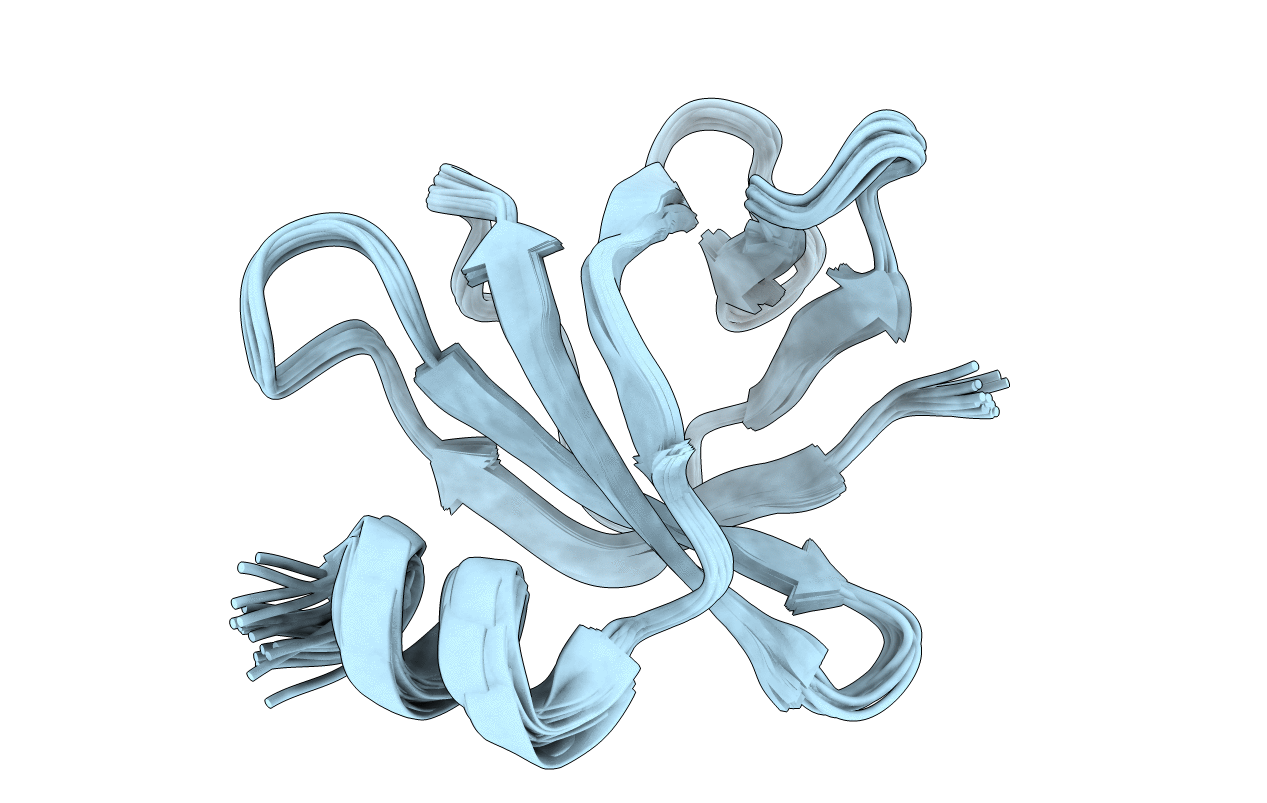
In bacteria, the two-component system (TCS) is the most prevalent for sensing and transducing the environmental signals into the cell. In Salmonella, the small basic protein PmrD is found to protect phospho-PmrA and prolong the expression of PmrA-activated genes. In contrast, Escherichia coli PmrD fails to protect phospho-PmrA. Here, we show that Klebsiella pneumoniae PmrD (KP-PmrD) can inhibit the dephosphrylation of phospho-PmrA, and the interaction between KP-PmrD and the N-terminal receiver domain of PmrA (PmrA(N)) is much stronger in the presence than in the absence of the phosphoryl analog beryllofluoride (BeF(3)(-)) (K(D)=1.74 ± 0.81 μM vs. K(D)=236 ± 48 μM). To better understand the molecular interactions involved, the solution structure of KP-PmrD was found to comprise six β-strands and a flexible C-terminal α-helix. Amide chemical shift perturbations of KP-PmrD in complex with BeF(3)(-)-activated PmrA(N) suggested that KP-PmrD may undergo a certain conformational rearrangement on binding to activated PmrA(N). Saturation transfer experiments revealed the binding surface to be located on one face of the β-barrel. This finding was further verified by in vivo polymyxin B susceptibility assay of the mutants of KP-PmrD. The phospho-PmrA recognition surface of KP-PmrD, which involves two KP-PmrD proteins in complex with an activated-PmrA(N) dimer, is suggested to be a contiguous patch consisting of Trp3, Trp4, Ser23, Leu26, Glu27, Met28, Thr46, Leu48, Ala49, Asp50, Ala51, Arg52, Ile65, Asn67, Ala68, Thr69, His70, Tyr71, Ser73 and Glu74. Our study furthers the understanding of how PmrD protects phopho-PmrA in the PmrAB TCS.