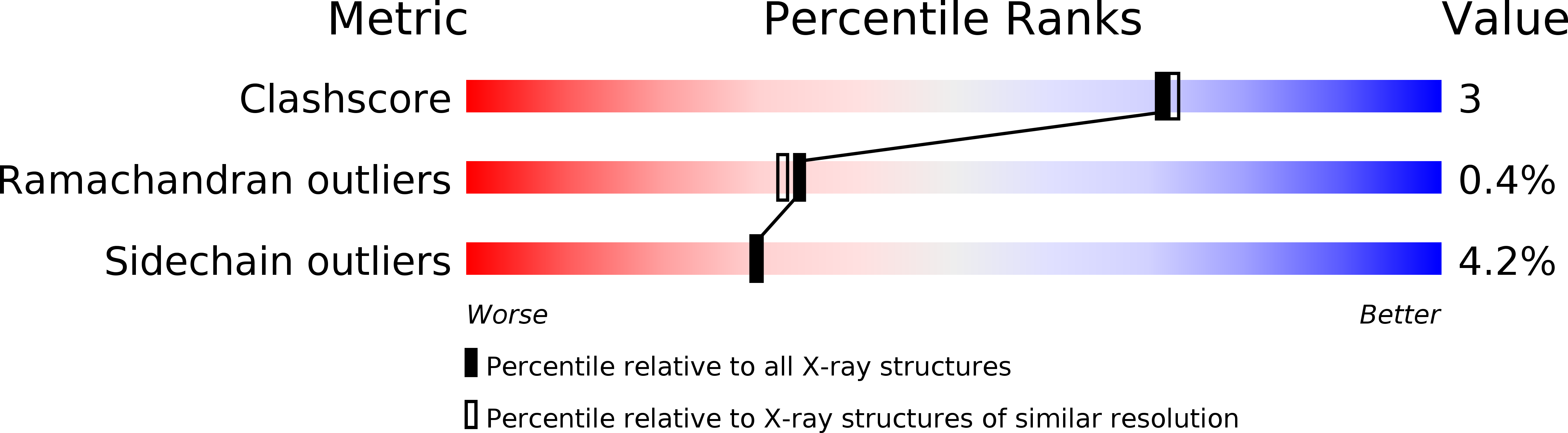
Deposition Date
1994-01-07
Release Date
1995-02-07
Last Version Date
2025-03-26
Entry Detail
PDB ID:
2RMA
Keywords:
Title:
Crystal structures of cyclophilin A complexed with cyclosporin A and N-methyl-4-[(E)-2-butenyl]-4,4-dimethylthreonine cyclosporin A
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
TOLYPOCLADIUM INFLATUM (Taxon ID: 29910)
TOLYPOCLADIUM INFLATUM (Taxon ID: 29910)
Method Details:
Experimental Method:
Resolution:
2.10 Å
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 1 21 1