Deposition Date
2007-10-18
Release Date
2008-03-18
Last Version Date
2023-08-30
Entry Detail
PDB ID:
2RKX
Keywords:
Title:
The 3D structure of chain D, cyclase subunit of imidazoleglycerol_evolvedcerolphosphate synthase
Method Details:
Experimental Method:
Resolution:
2.25 Å
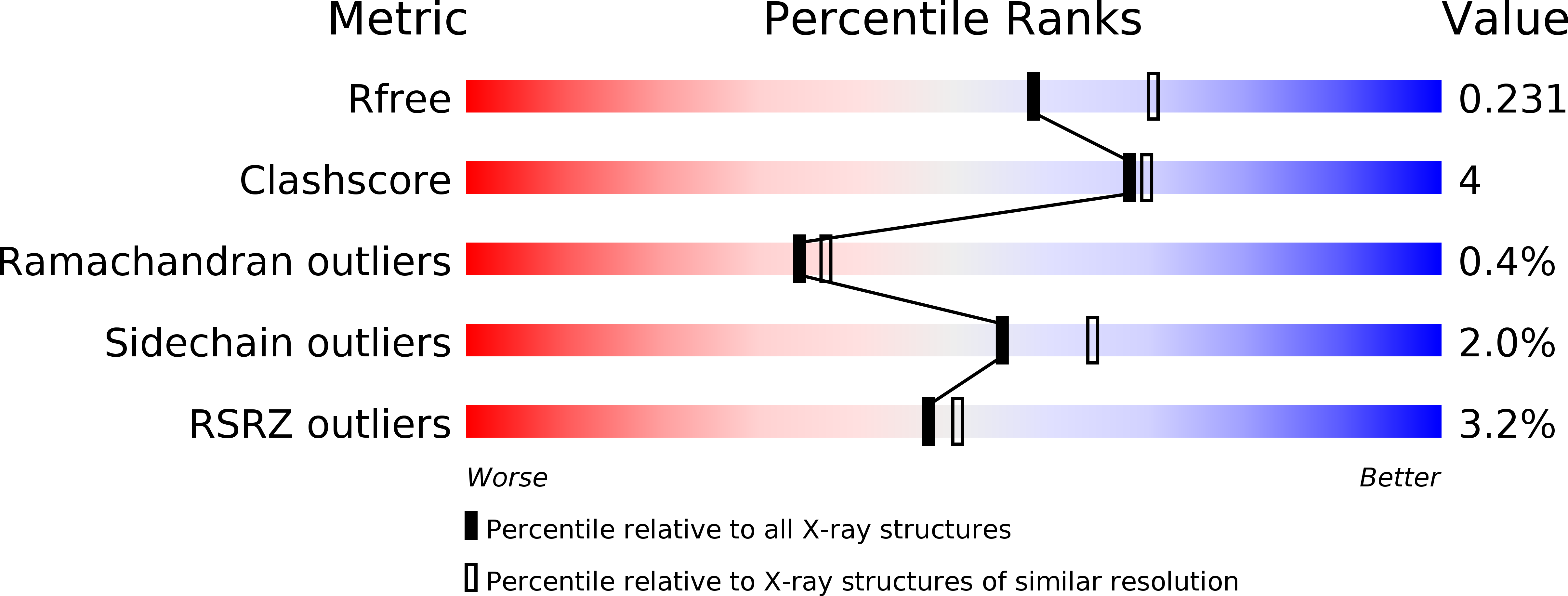
R-Value Free:
0.23
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 61 2 2