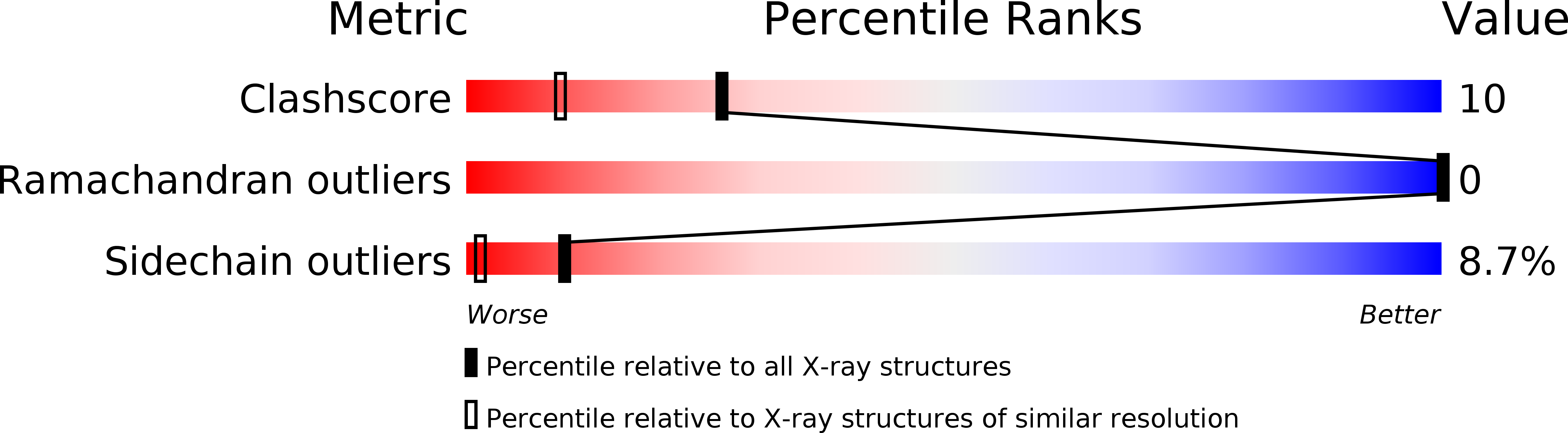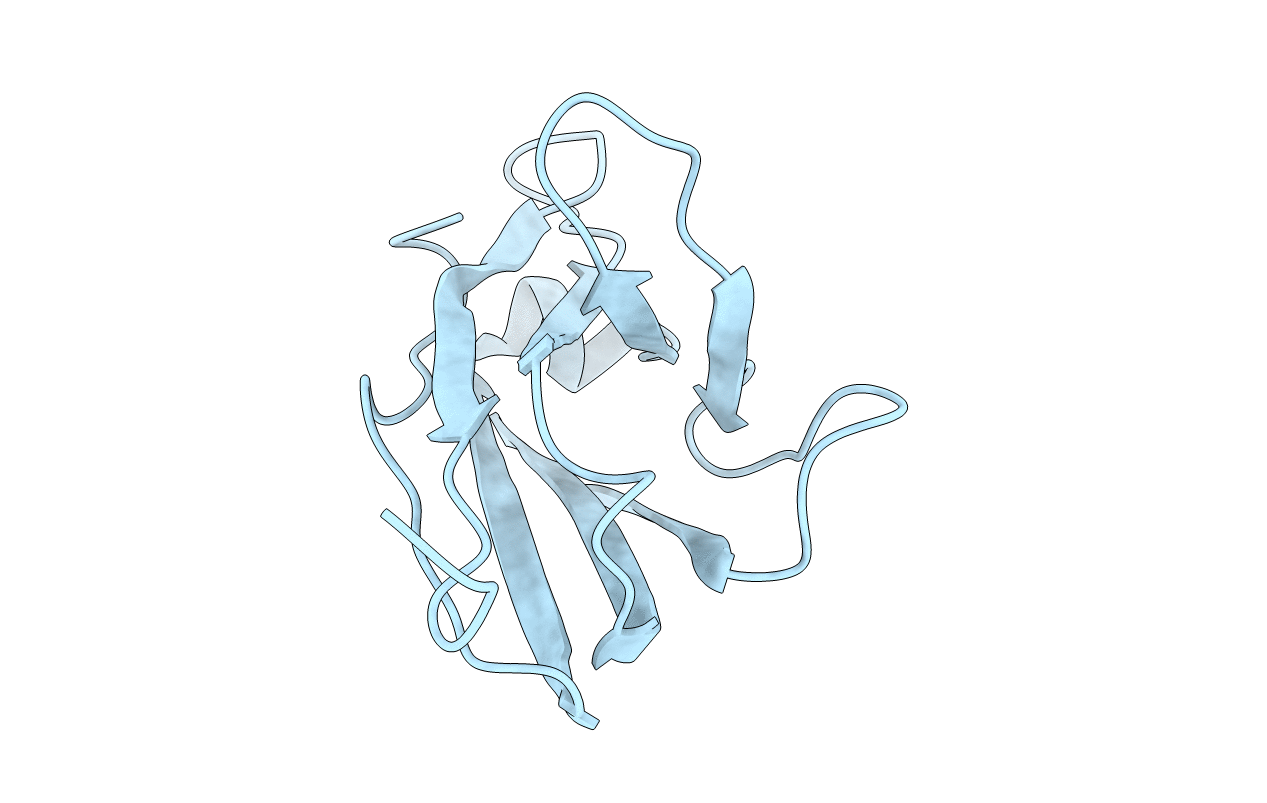
Deposition Date
1983-06-13
Release Date
1983-09-15
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2RHE
Keywords:
Title:
STRUCTURE OF A NOVEL BENCE-JONES PROTEIN (RHE) FRAGMENT AT 1.6 ANGSTROMS RESOLUTION
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details: