Deposition Date
2007-09-06
Release Date
2007-12-25
Last Version Date
2024-02-21
Entry Detail
PDB ID:
2R6P
Keywords:
Title:
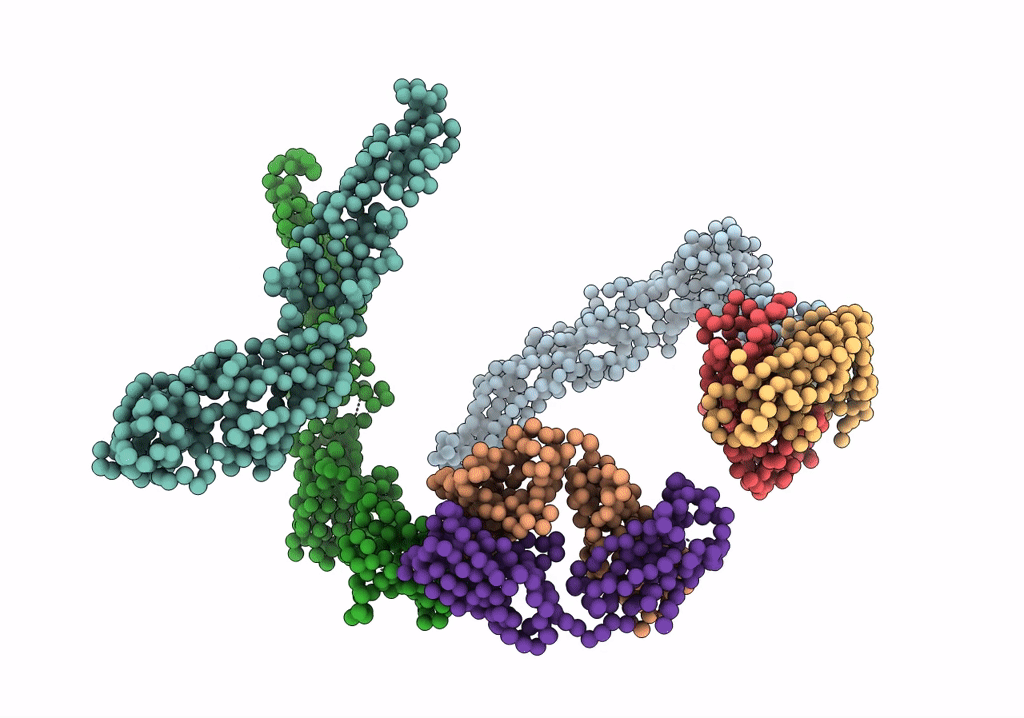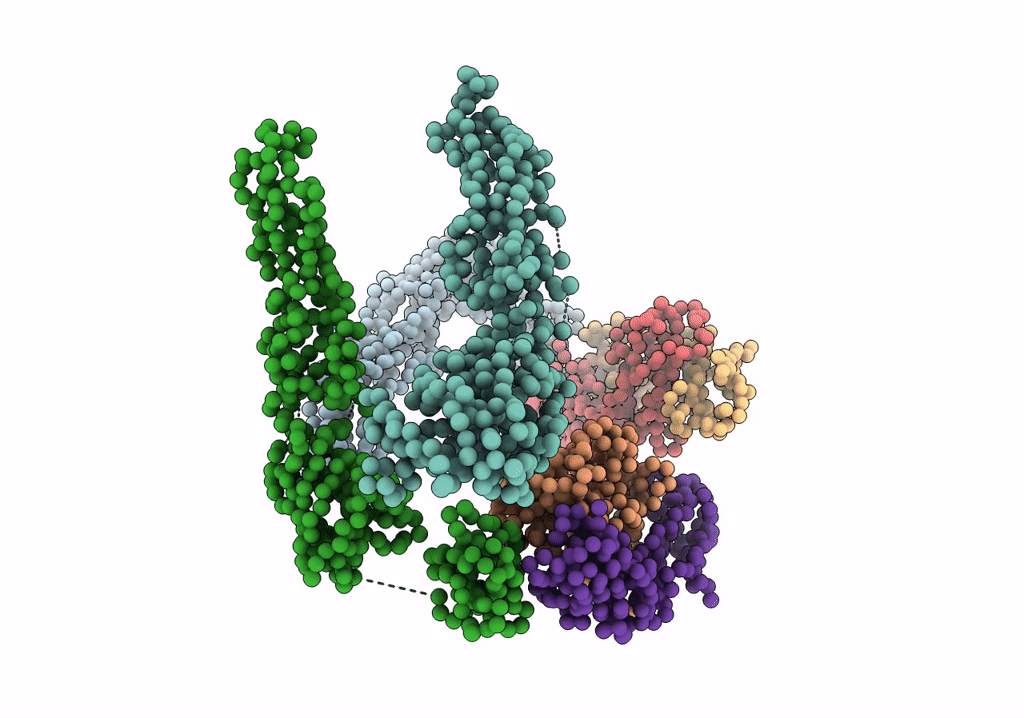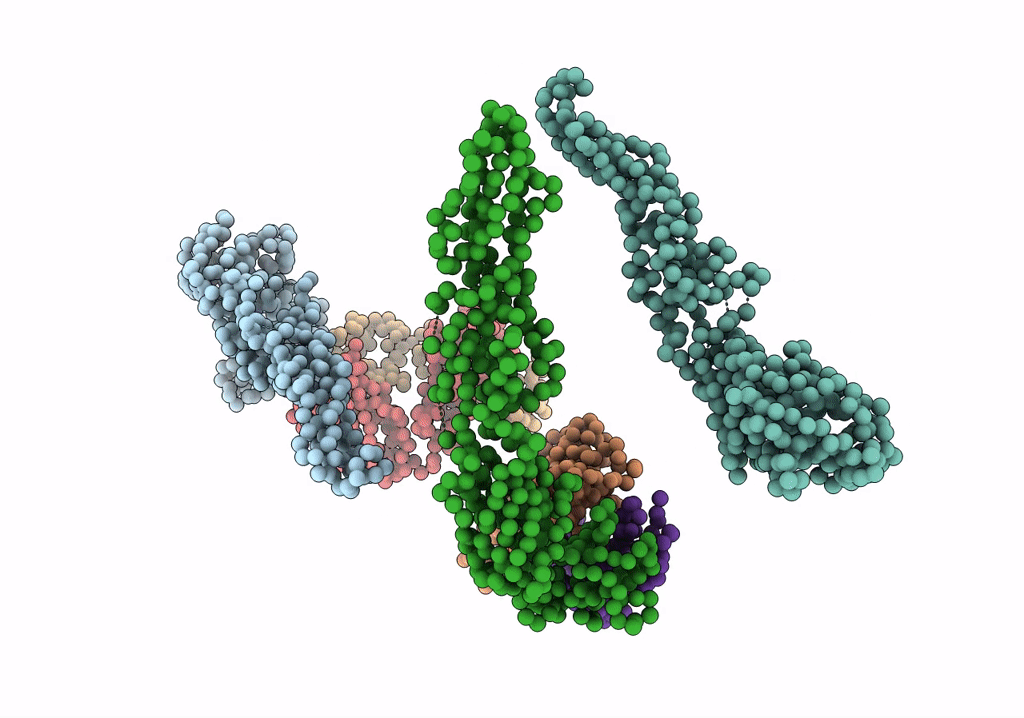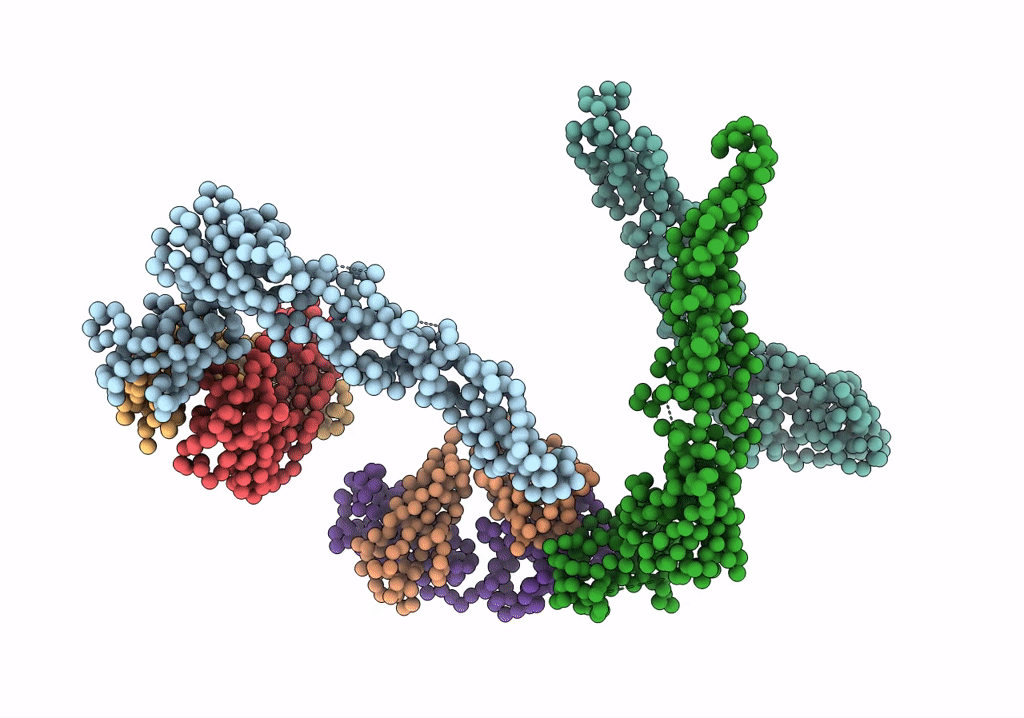
Fit of E protein and Fab 1A1D-2 into 24 angstrom resolution cryoEM map of Fab complexed with dengue 2 virus.
Biological Source:
Source Organism(s):
Dengue virus 2 Puerto Rico/PR159-S1/1969 (Taxon ID: 11066)
Mus musculus (Taxon ID: 10090)
Mus musculus (Taxon ID: 10090)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
24.00 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE