Deposition Date
2007-08-15
Release Date
2007-11-13
Last Version Date
2023-10-25
Entry Detail
PDB ID:
2QYJ
Keywords:
Title:
Crystal structure of a designed full consensus ankyrin
Method Details:
Experimental Method:
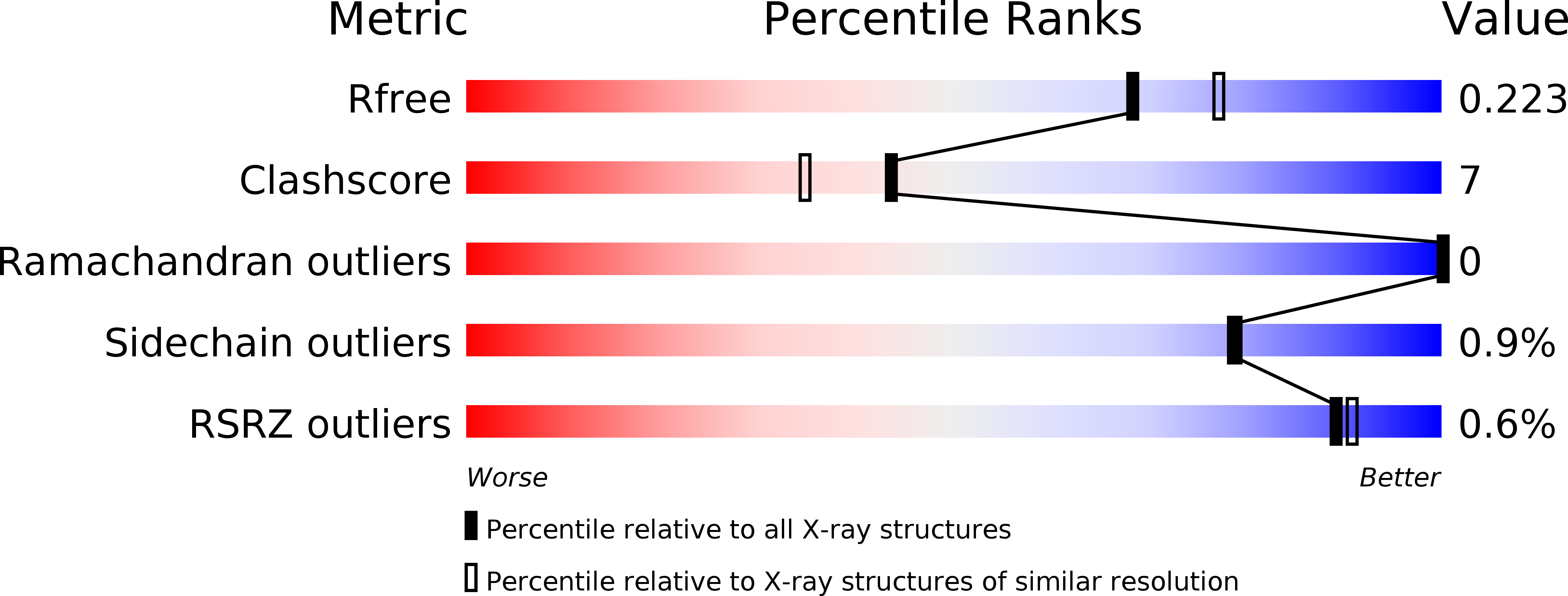
Resolution:
2.05 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 61