Deposition Date
2007-06-14
Release Date
2007-08-14
Last Version Date
2023-08-30
Entry Detail
PDB ID:
2QA2
Keywords:
Title:
Crystal structure of CabE, an aromatic hydroxylase from angucycline biosynthesis, determined to 2.7 A resolution
Biological Source:
Source Organism(s):
Streptomyces (Taxon ID: 1883)
Expression System(s):
Method Details:
Experimental Method:
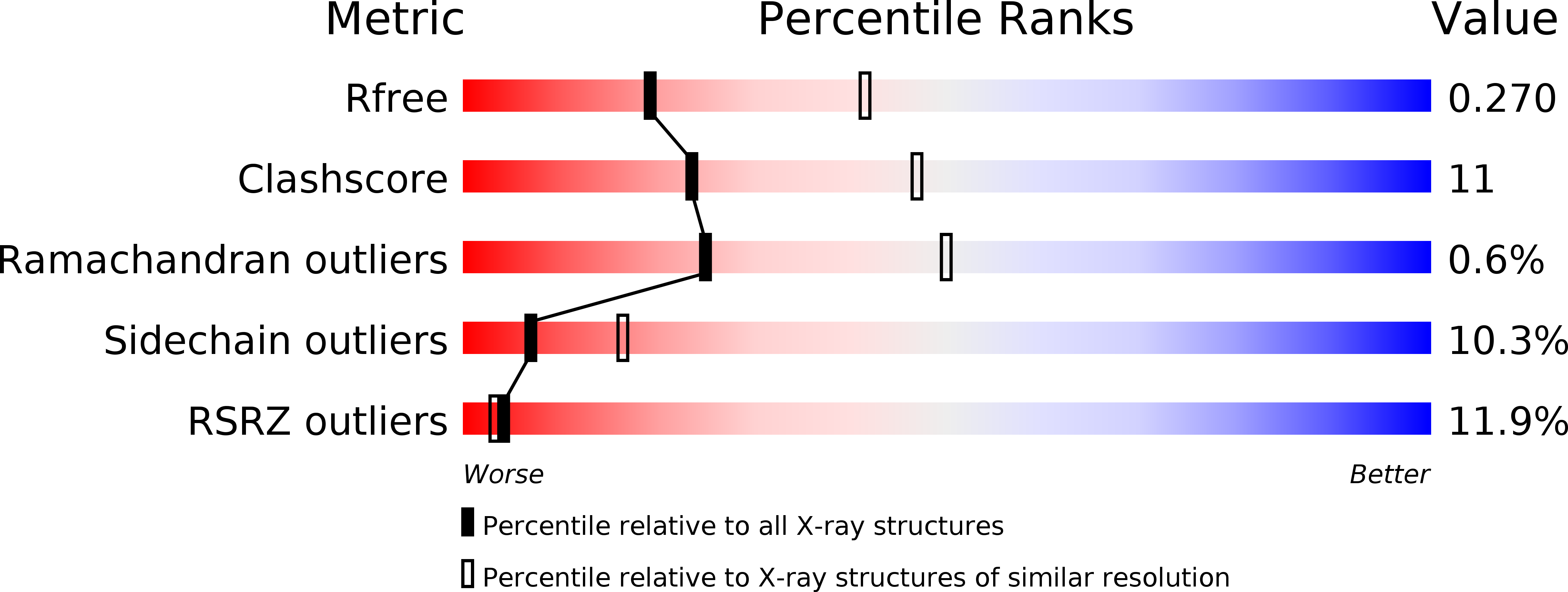
Resolution:
2.70 Å
R-Value Free:
0.27
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 65 2 2