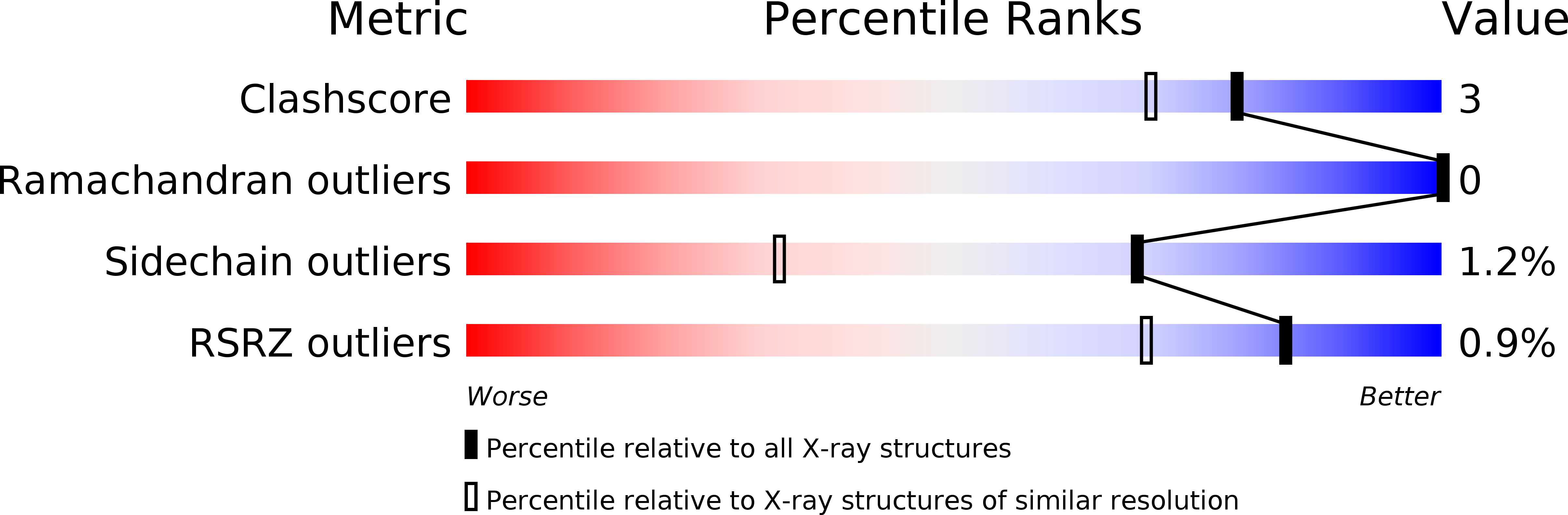Deposition Date
1998-10-02
Release Date
1998-10-07
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2PVB
Keywords:
Title:
PIKE PARVALBUMIN (PI 4.10) AT LOW TEMPERATURE (100K) AND ATOMIC RESOLUTION (0.91 A).
Biological Source:
Source Organism(s):
Esox lucius (Taxon ID: 8010)
Method Details:
Experimental Method:
Resolution:
0.91 Å
R-Value Free:
0.13
R-Value Observed:
0.10
Space Group:
P 21 21 21