Deposition Date
2007-05-04
Release Date
2008-03-18
Last Version Date
2023-08-30
Entry Detail
PDB ID:
2PR9
Keywords:
Title:
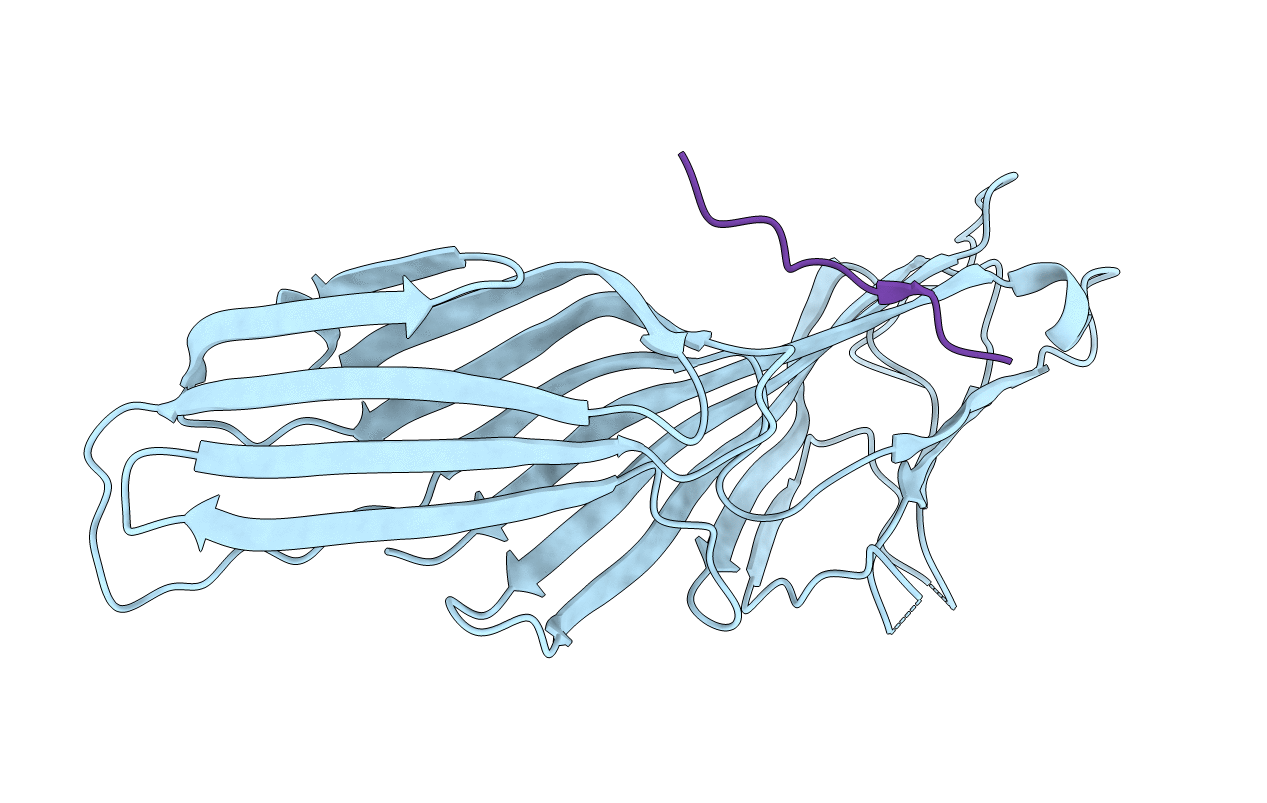
Mu2 adaptin subunit (AP50) of AP2 adaptor (second domain), complexed with GABAA receptor-gamma2 subunit-derived internalization peptide DEEYGYECL
Biological Source:
Source Organism(s):
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
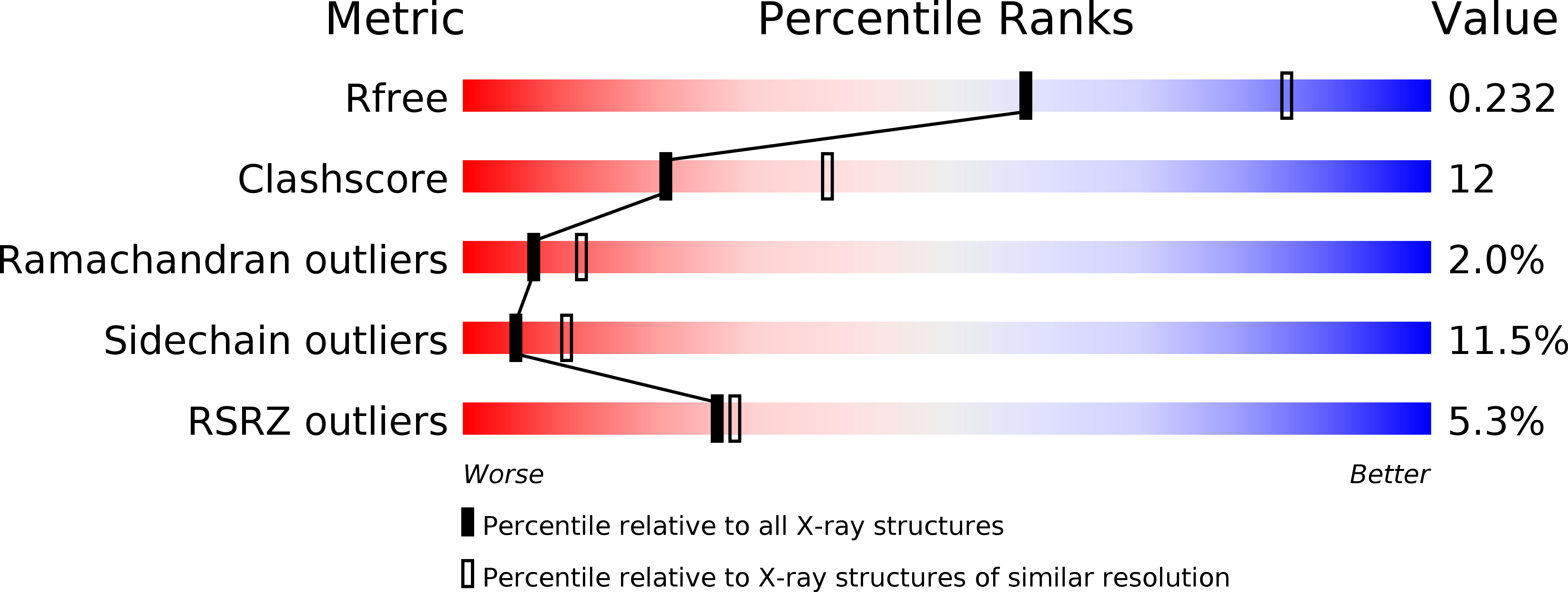
Resolution:
2.51 Å
R-Value Free:
0.24
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 64