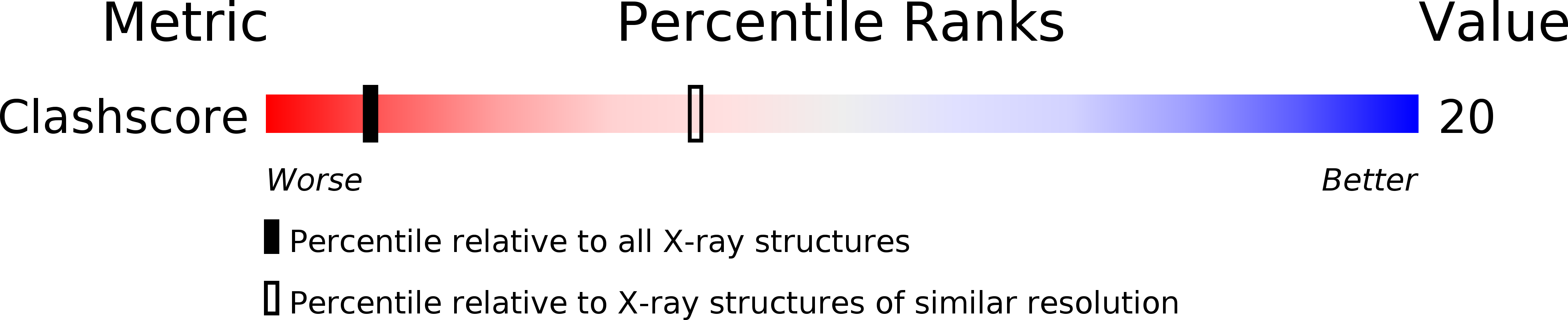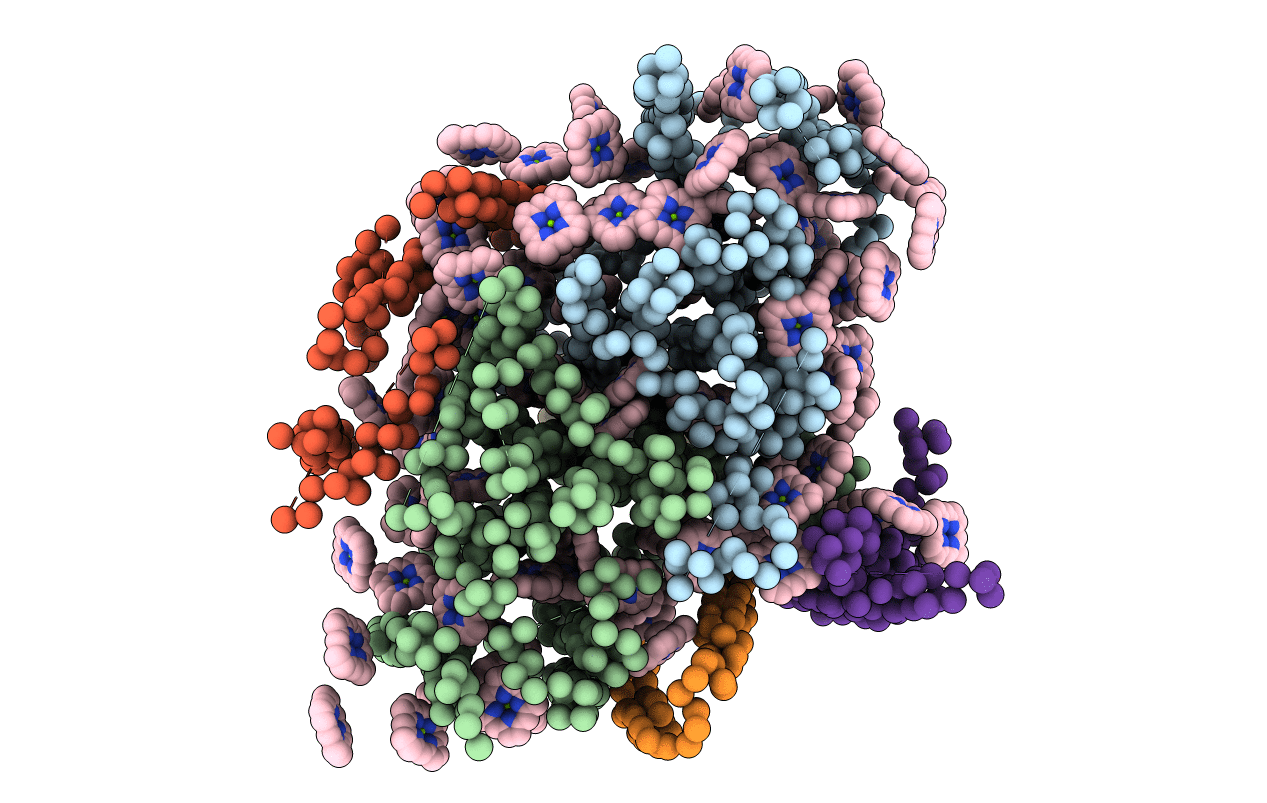
Deposition Date
1997-05-27
Release Date
1998-05-27
Last Version Date
2024-02-21
Entry Detail
PDB ID:
2PPS
Keywords:
Title:
PHOTOSYNTHETIC REACTION CENTER AND CORE ANTENNA SYSTEM (TRIMERIC), ALPHA CARBON ONLY
Biological Source:
Source Organism(s):
Synechococcus elongatus (Taxon ID: 32046)
Method Details: