Deposition Date
2007-04-25
Release Date
2008-03-18
Last Version Date
2023-08-30
Entry Detail
PDB ID:
2PO1
Keywords:
Title:
Crystal structure of the P. abyssi exosome RNase PH ring complexed with a single stranded 10-mer poly(A) RNA
Biological Source:
Source Organism(s):
Pyrococcus abyssi (Taxon ID: 29292)
Expression System(s):
Method Details:
Experimental Method:
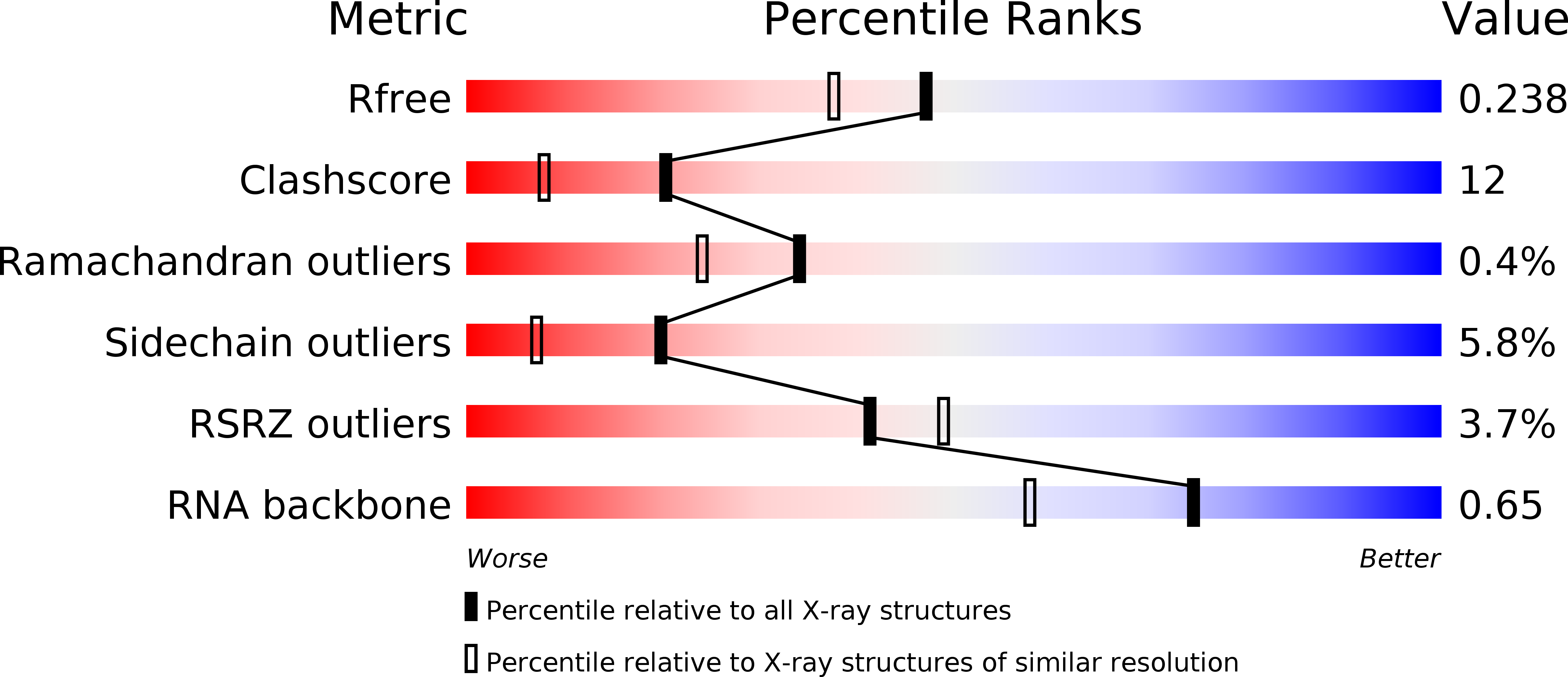
Resolution:
1.94 Å
R-Value Free:
0.24
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
P 3 2 1