Deposition Date
2007-03-20
Release Date
2007-05-22
Last Version Date
2023-08-30
Entry Detail
PDB ID:
2P7F
Keywords:
Title:
The Novel Use of a 2',5'-Phosphodiester Linkage as a Reaction Intermediate at the Active Site of a Small Ribozyme
Method Details:
Experimental Method:
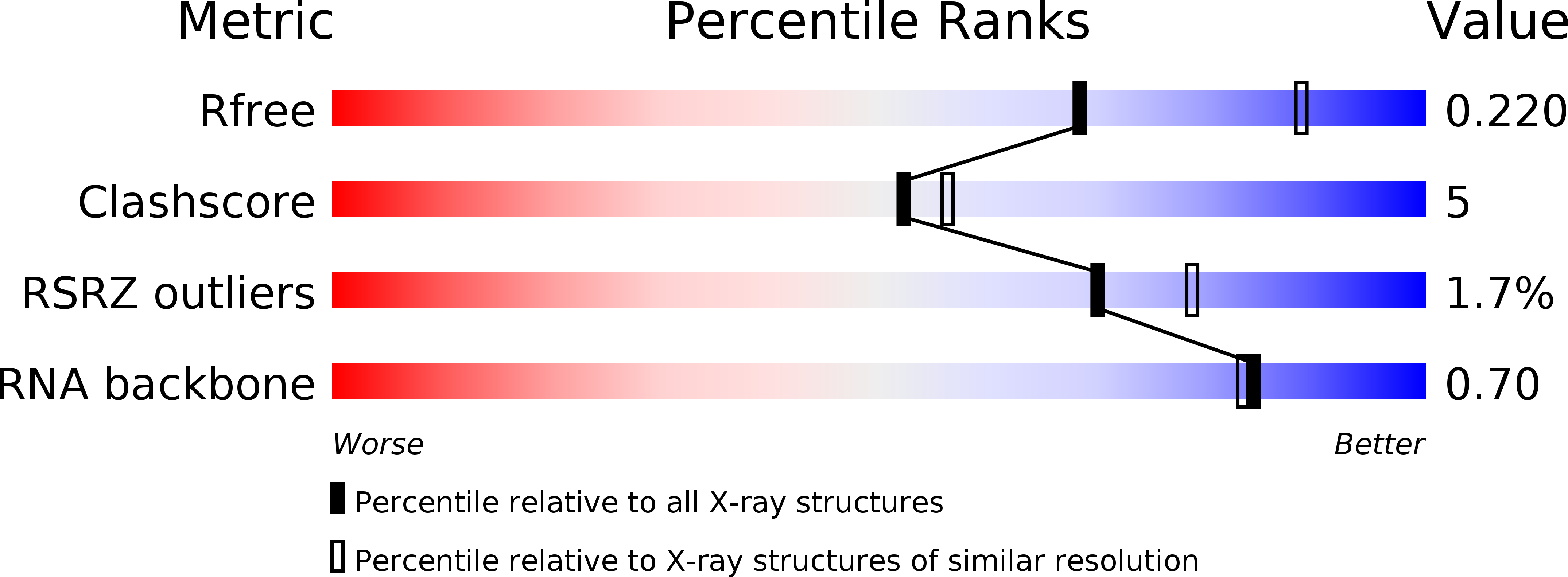
Resolution:
2.35 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 61 2 2