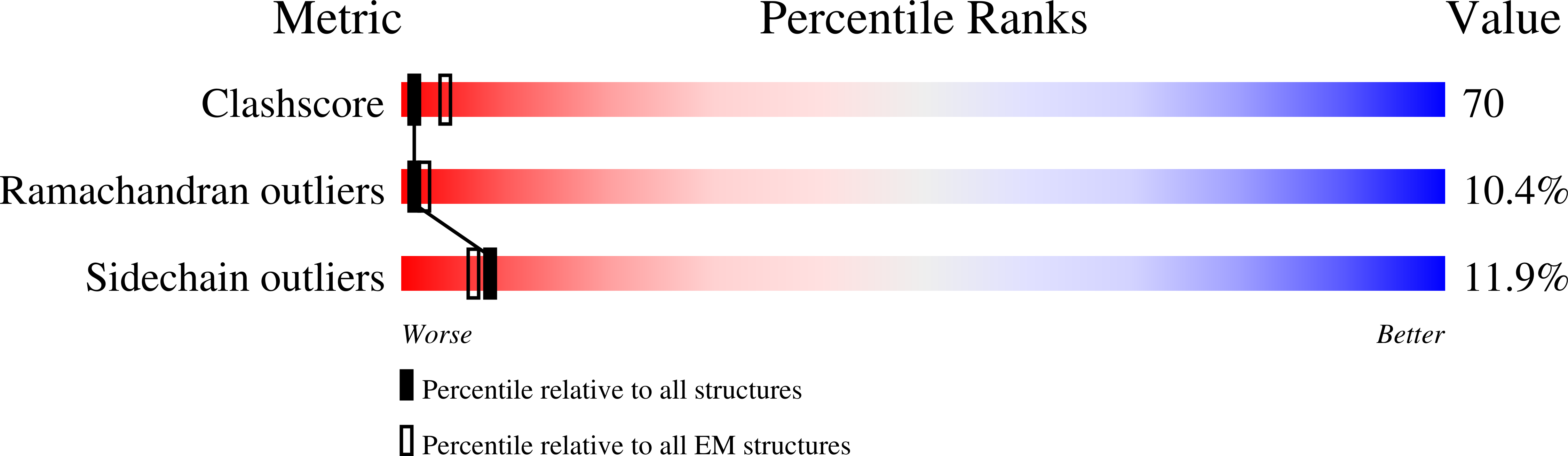
Deposition Date
2007-03-12
Release Date
2008-07-08
Last Version Date
2024-02-21
Entry Detail
PDB ID:
2P4N
Keywords:
Title:
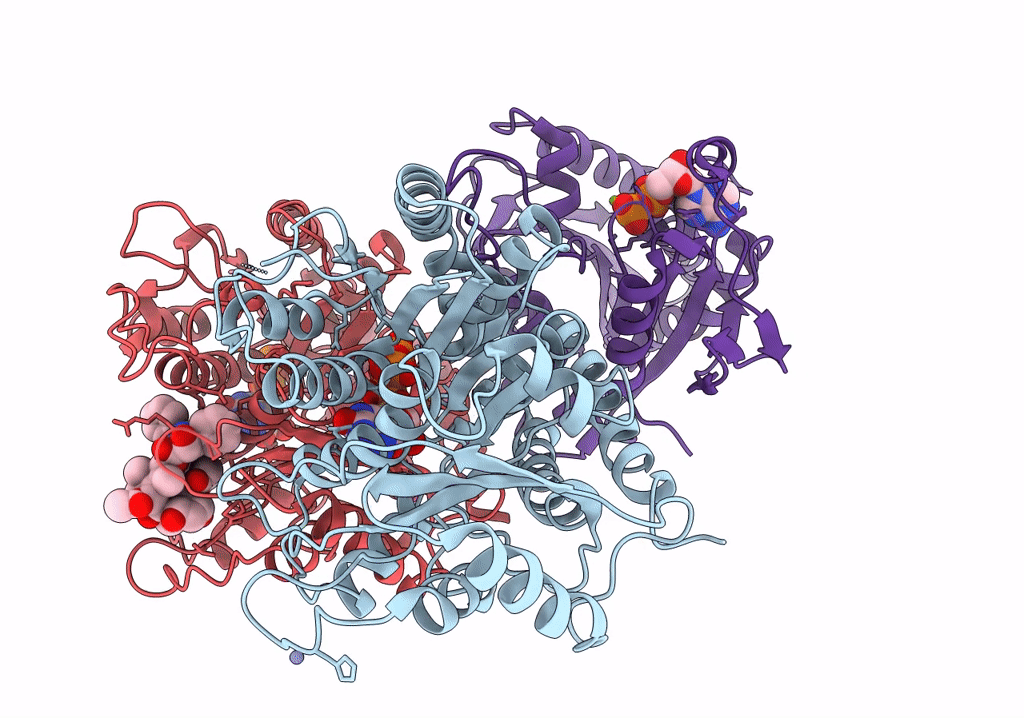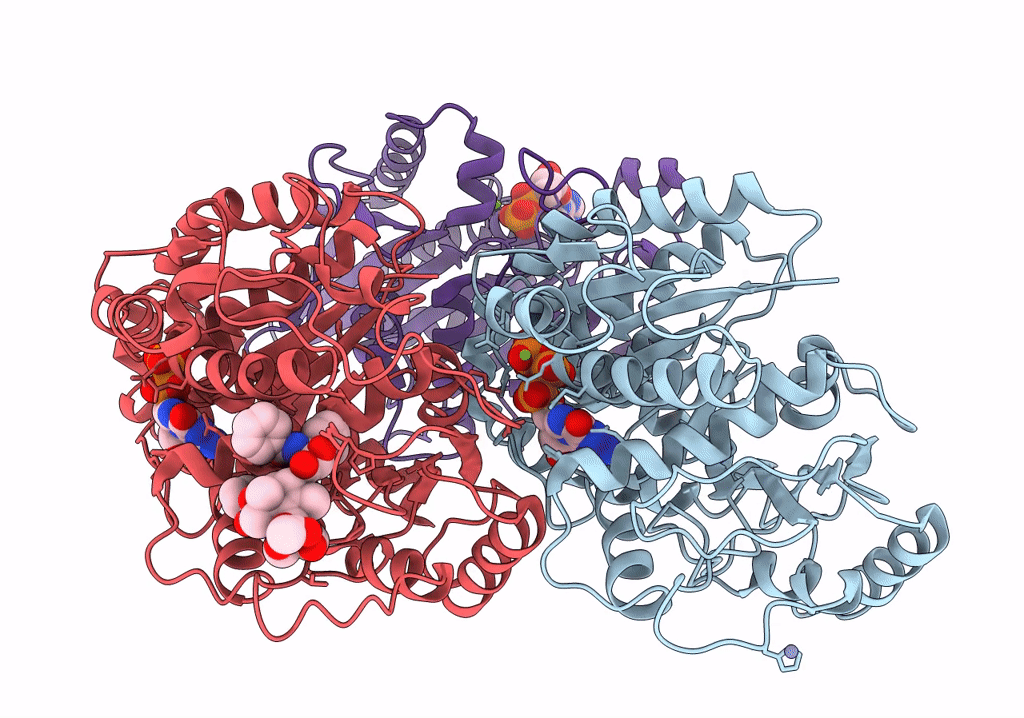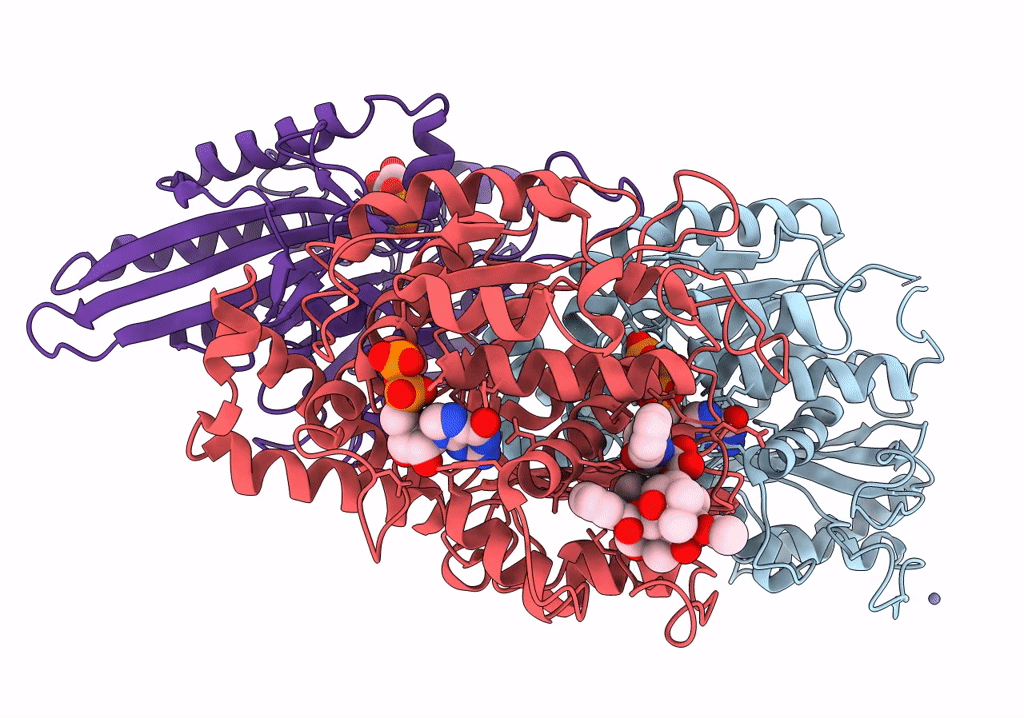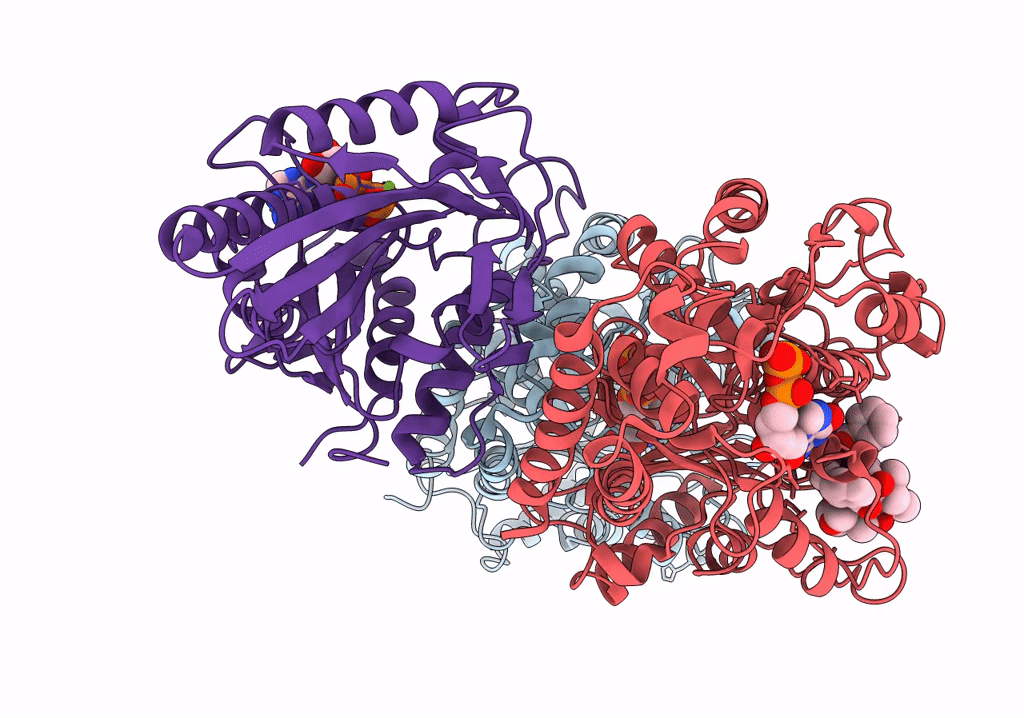
Human Monomeric Kinesin (1BG2) and Bovine Tubulin (1JFF) Docked into the 9-Angstrom Cryo-EM Map of Nucleotide-Free Kinesin Complexed to the Microtubule
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: )
Method Details:
Experimental Method:
Resolution:
9.00 Å
Aggregation State:
FILAMENT
Reconstruction Method:
SINGLE PARTICLE