Deposition Date
2007-03-09
Release Date
2008-01-22
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2P3T
Keywords:
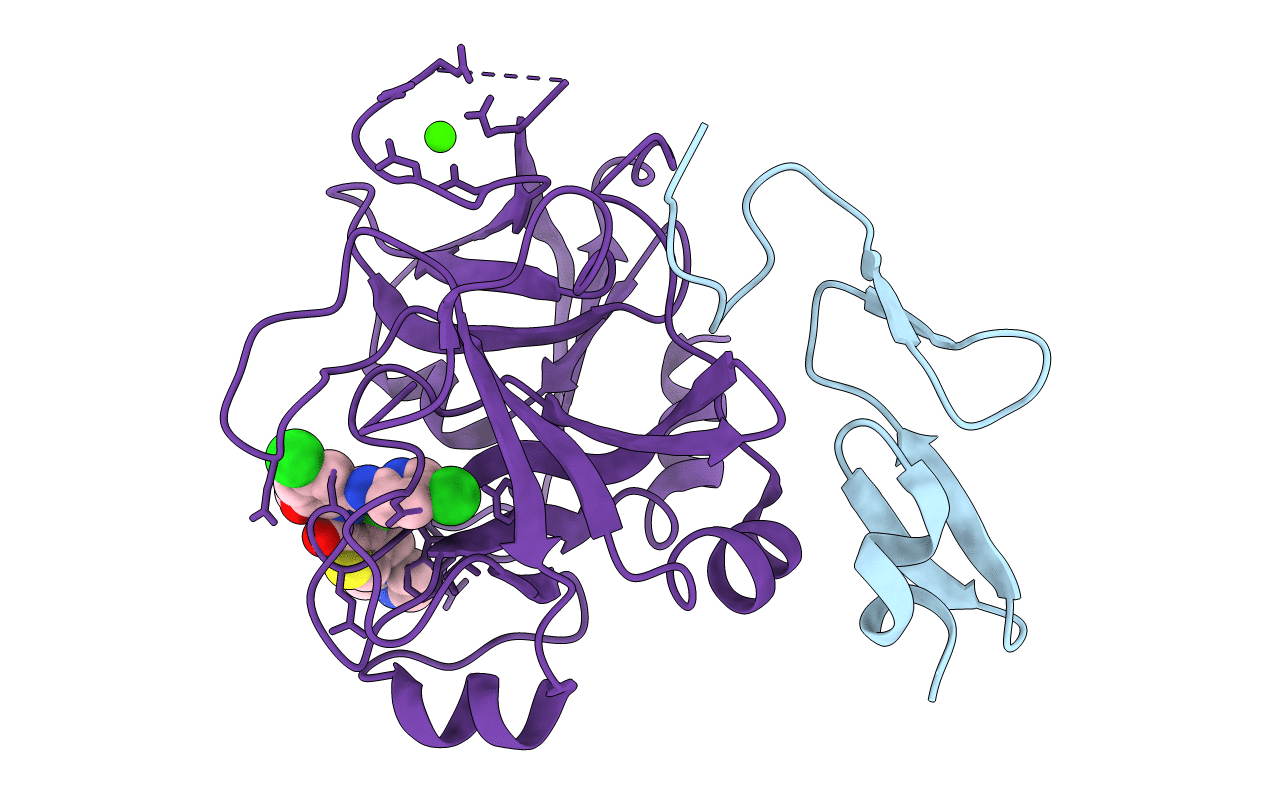
Title:
Crystal structure of human factor XA complexed with 3-Chloro-4-(2-methylamino-imidazol-1-ylmethyl)-thiophene-2-carboxylic acid [4-chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)-6-methoxy-phenyl]-amide
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
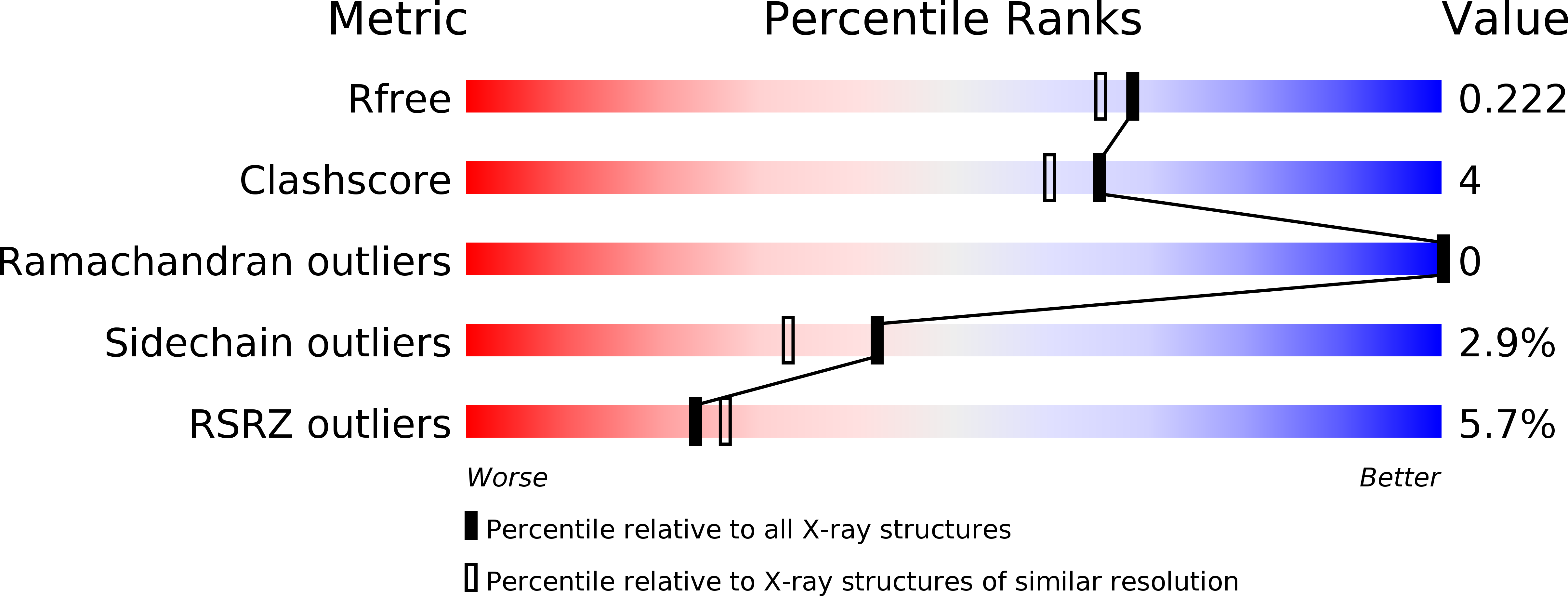
Resolution:
1.92 Å
R-Value Free:
0.21
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21