Deposition Date
2007-02-28
Release Date
2007-11-13
Last Version Date
2024-04-03
Entry Detail
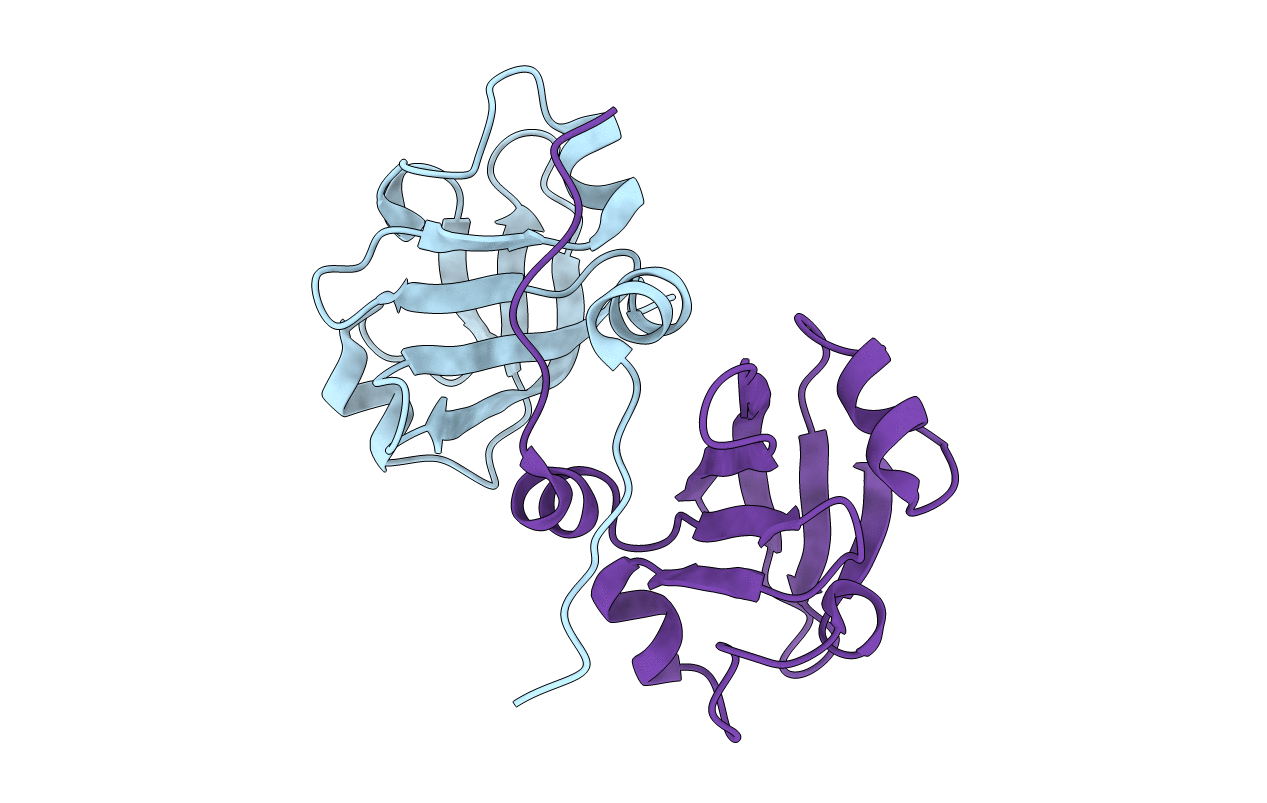
PDB ID:
2P04
Keywords:
Title:
2.1 Ang structure of the dimerized PAS domain of signal transduction histidine kinase from Nostoc punctiforme PCC 73102 with homology to the H-NOXA/H-NOBA domain of the soluble guanylyl cyclase
Biological Source:
Source Organism(s):
Nostoc punctiforme (Taxon ID: 63737)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.11 Å
R-Value Free:
0.26
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
C 1 2 1


