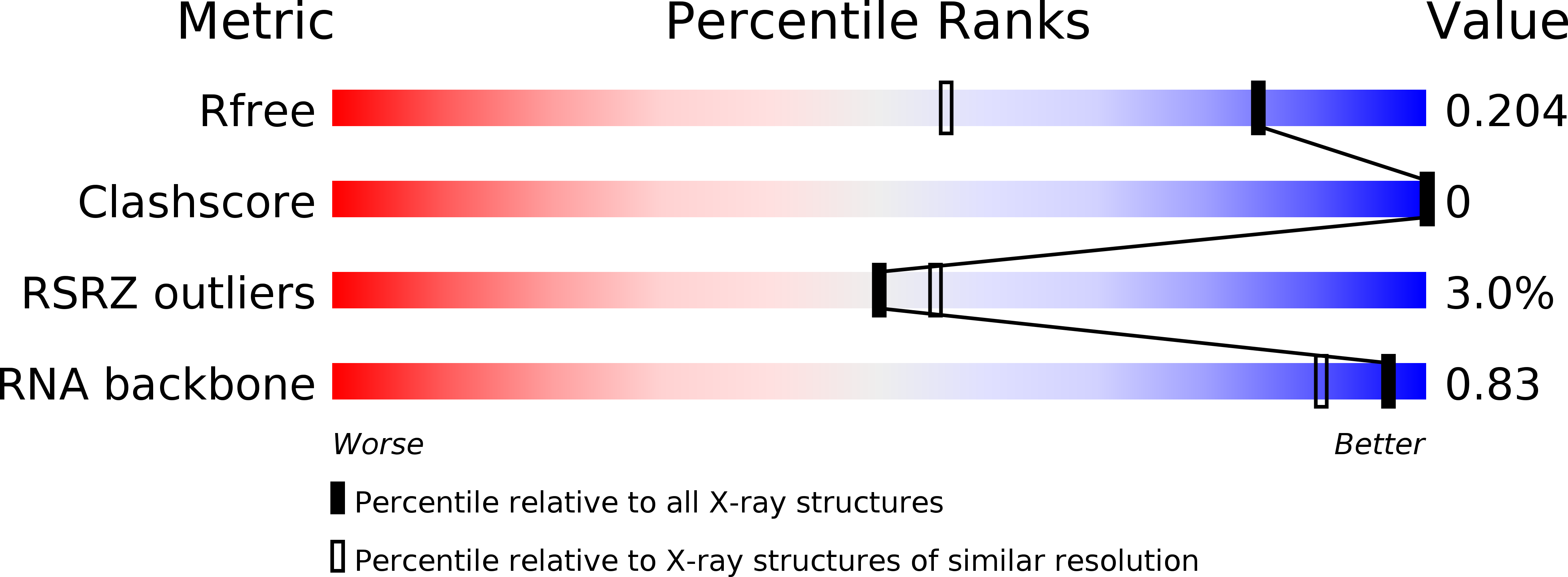
Deposition Date
2006-12-28
Release Date
2007-02-13
Last Version Date
2024-04-03
Entry Detail
PDB ID:
2OE5
Keywords:
Title:
1.5 A X-ray crystal structure of Apramycin complex with RNA fragment GGCGUCGCUAGUACCG/GGUACUAAAAGUCGCCC containing the human ribosomal decoding A site: RNA construct with 3'-overhang
Method Details:
Experimental Method:
Resolution:
1.51 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
P 21 21 21