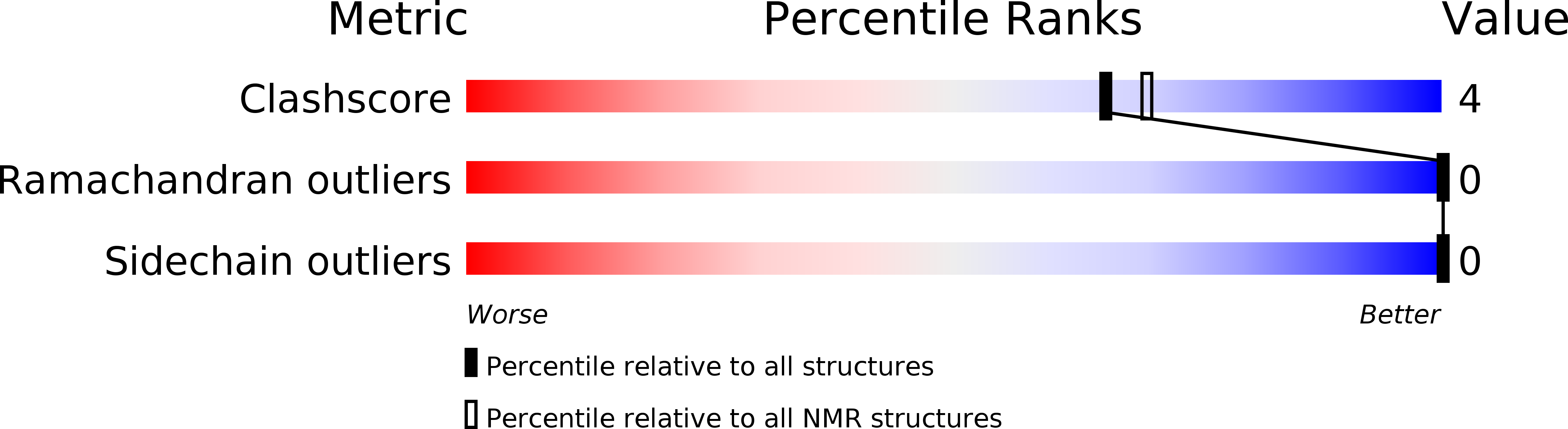
Deposition Date
2016-01-24
Release Date
2016-06-29
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2NB5
Keywords:
Title:
NMR solution structure of PawS Derived Peptide 9 (PDP-9)
Biological Source:
Source Organism(s):
Perymenium macrocephalum (Taxon ID: 183067)
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
structures with acceptable covalent geometry