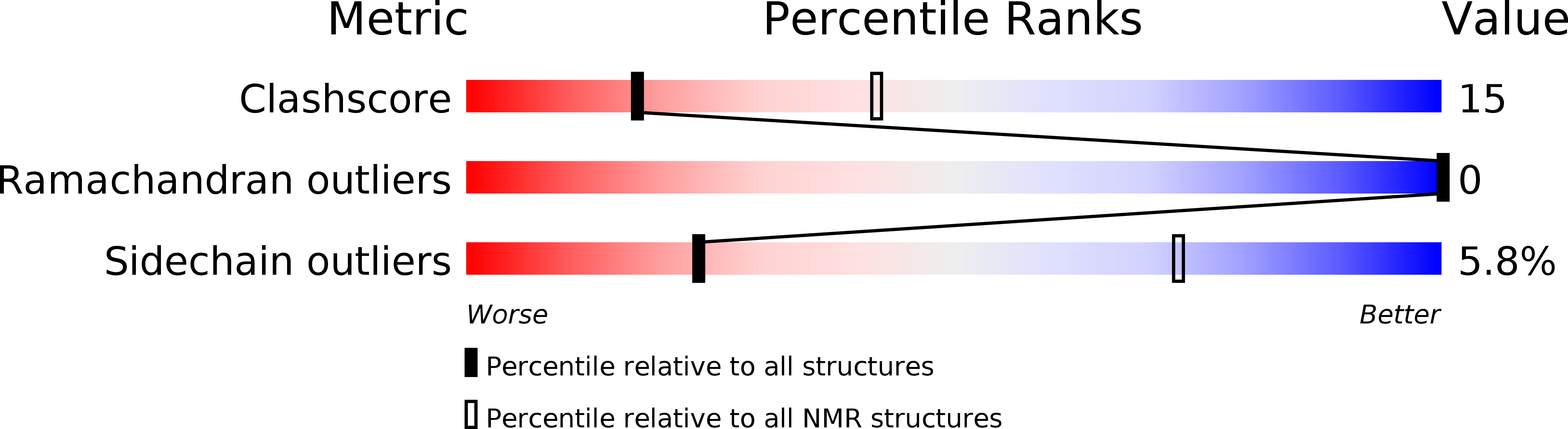
Deposition Date
2015-12-21
Release Date
2016-07-06
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2NA9
Keywords:
Title:
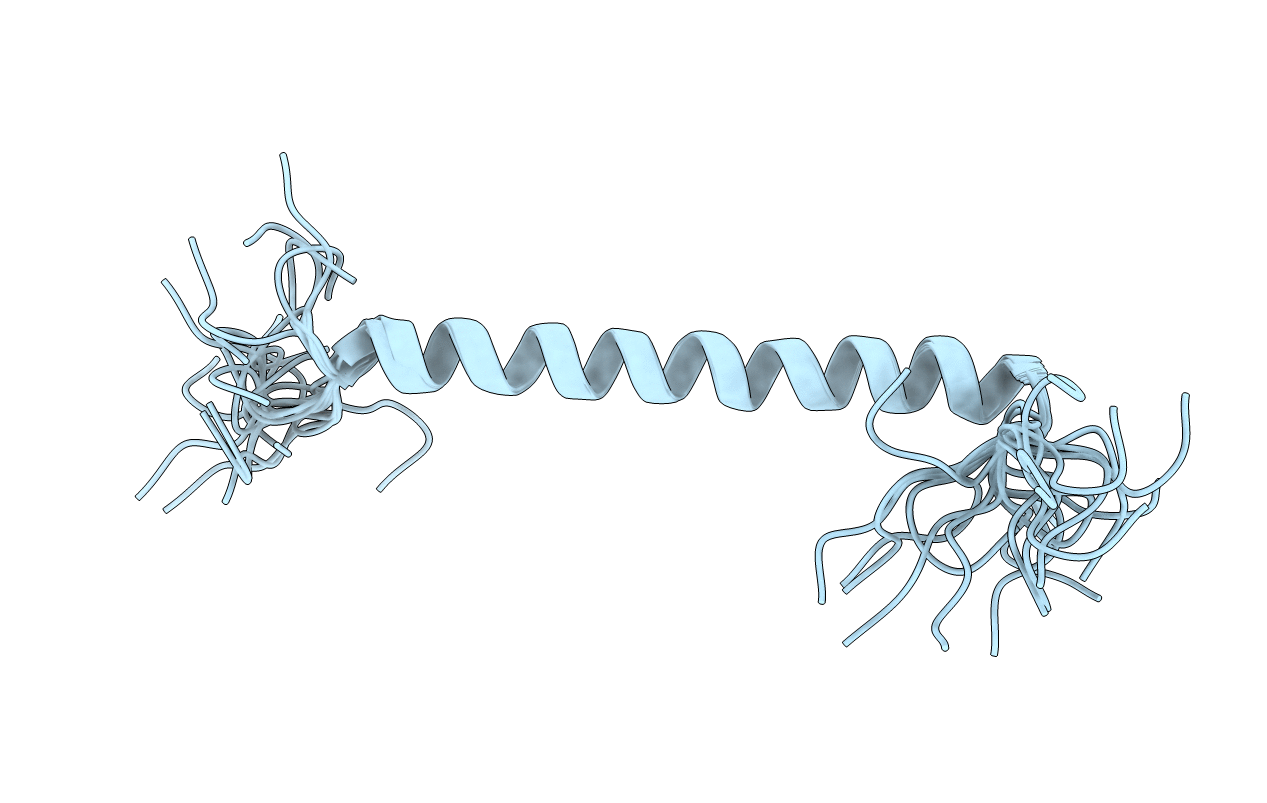
Transmembrane Structure of the P441A Mutant of the Cytokine Receptor Common Subunit beta
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
21
Conformers Submitted:
21
Selection Criteria:
all calculated structures submitted