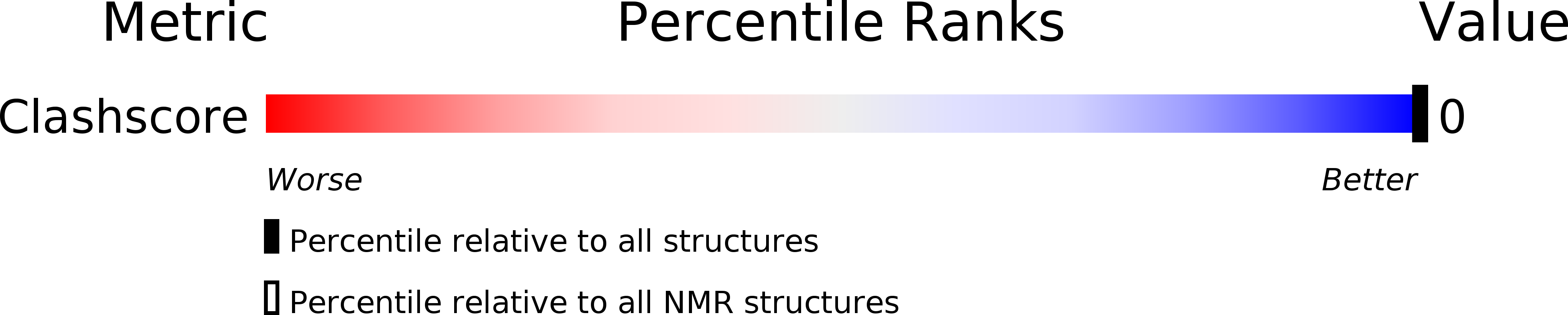
Deposition Date
2015-06-17
Release Date
2015-07-01
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2N4A
Keywords:
Title:
EC-NMR Structure of Ralstonia metallidurans Rmet_5065 Determined by Combining Evolutionary Couplings (EC) and Sparse NMR Data. Northeast Structural Genomics Consortium target CrR115
Biological Source:
Source Organism(s):
Cupriavidus metallidurans CH34 (Taxon ID: 266264)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
lowest energy