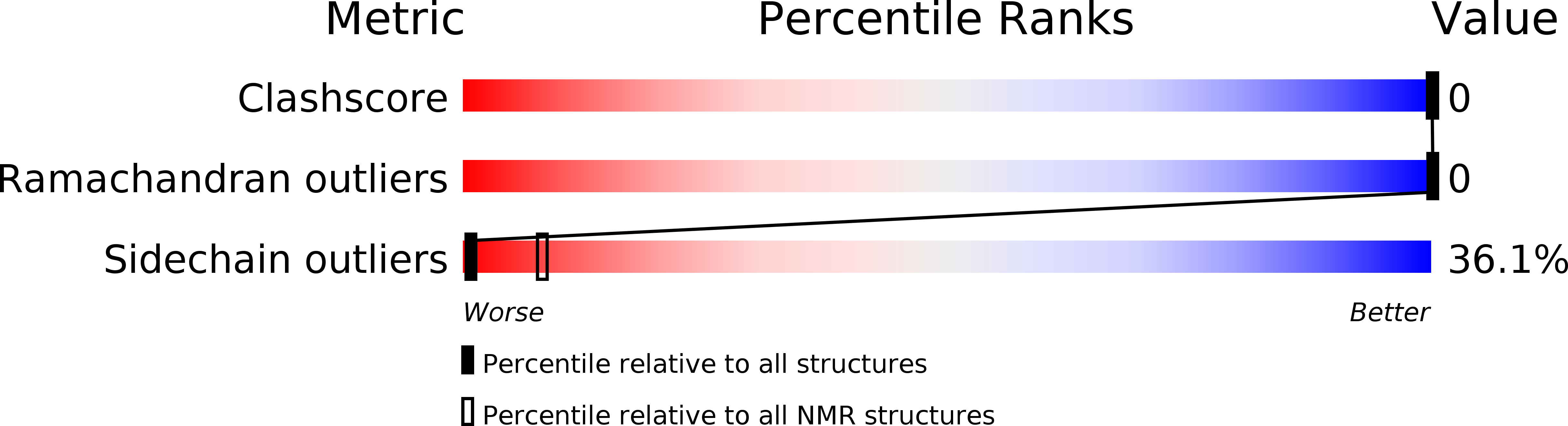
Deposition Date
2015-01-27
Release Date
2015-04-22
Last Version Date
2024-10-30
Entry Detail
PDB ID:
2MYM
Keywords:
Title:
Cullin3 - BTB interface: a novel target for stapled peptides
Biological Source:
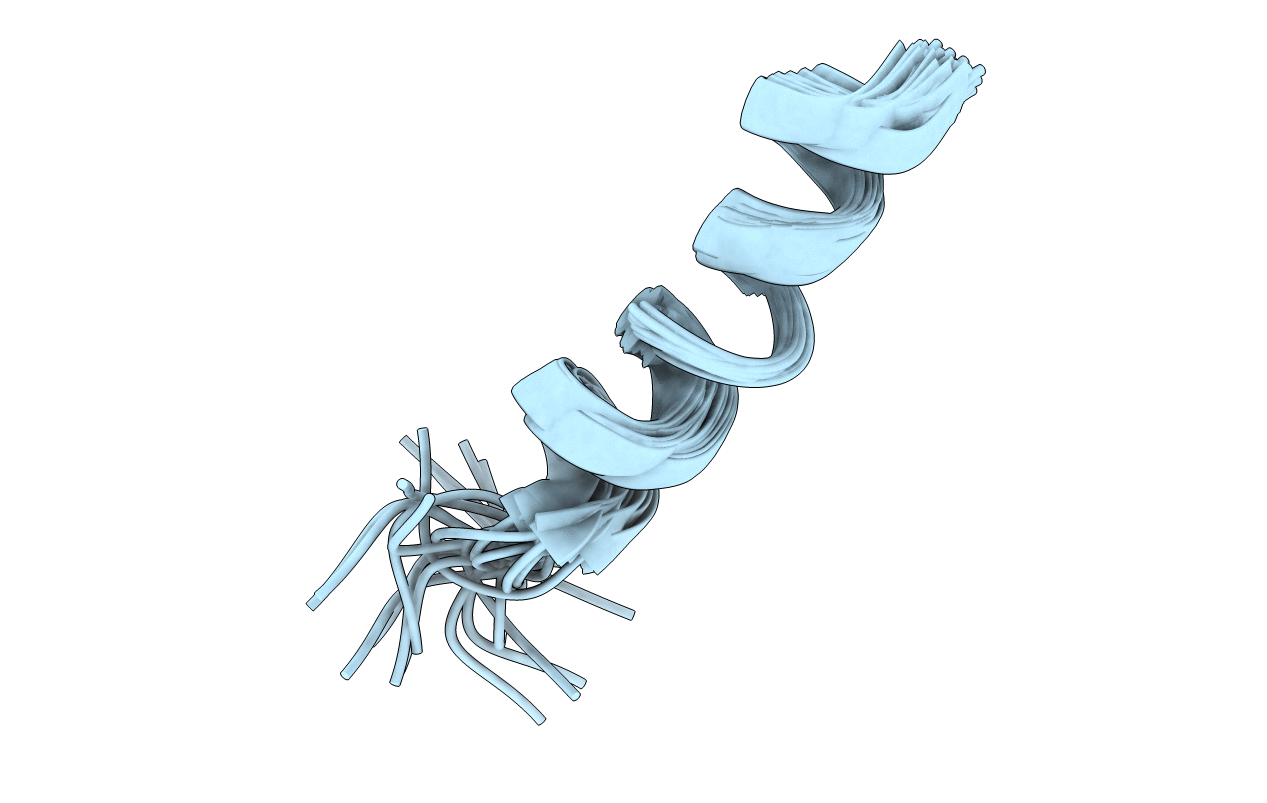
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy