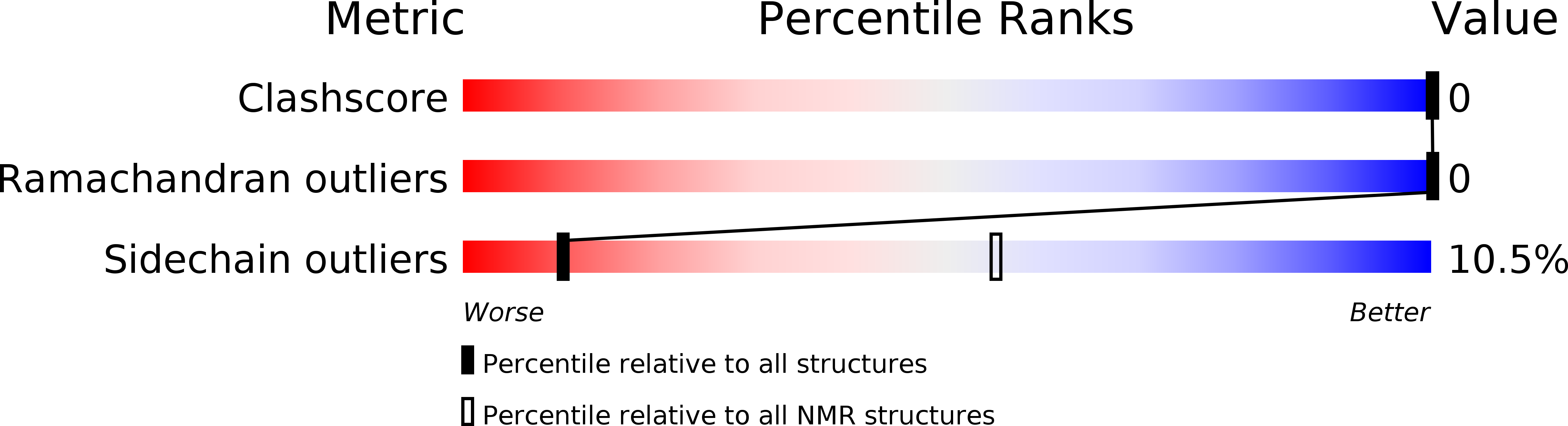Deposition Date
2014-09-04
Release Date
2014-12-17
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2MU6
Keywords:
Title:
3D structure determination of STARP peptides implicated in P. falciparum Invasion of hepatic cells
Biological Source:
Source Organism(s):
Plasmodium falciparum (Taxon ID: 5833)
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
1
Selection Criteria:
structures with the lowest energy