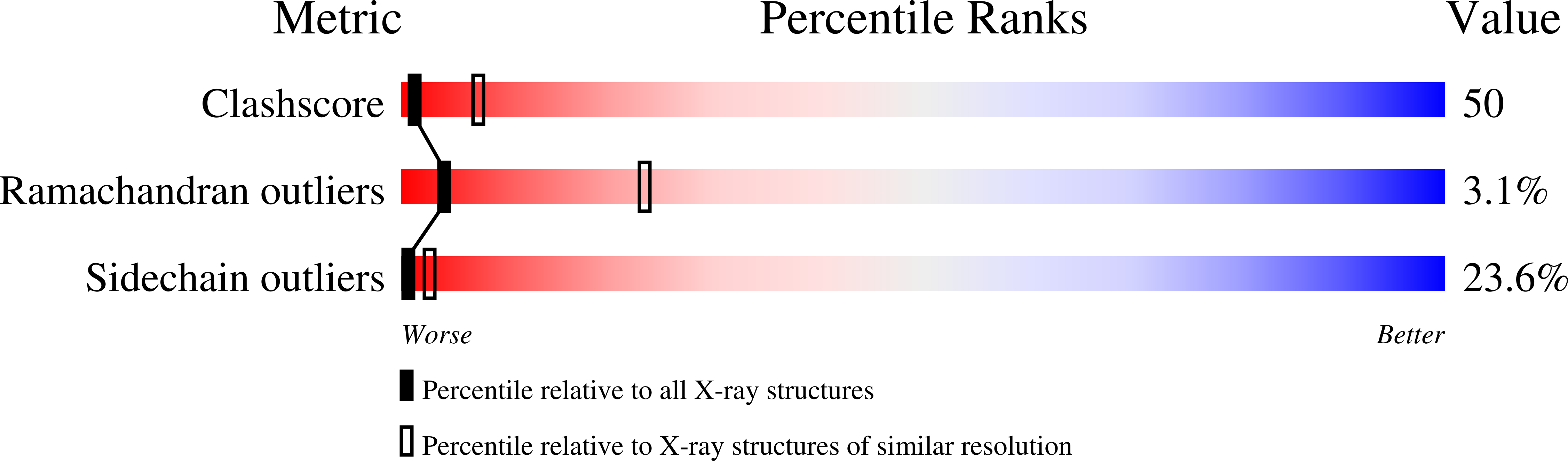Deposition Date
1997-12-19
Release Date
1998-04-15
Last Version Date
2024-05-22
Entry Detail
PDB ID:
2MSP
Keywords:
Title:
MAJOR SPERM PROTEIN, BETA ISOFORM, ENGINEERED C59S/T90C MUTANT, PUTATIVE SUBFILAMENT STRUCTURE, PH 8.5
Biological Source:
Source Organism(s):
Ascaris suum (Taxon ID: 6253)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.30 Å
R-Value Free:
0.26
R-Value Work:
0.21
R-Value Observed:
0.22
Space Group:
P 41 21 2