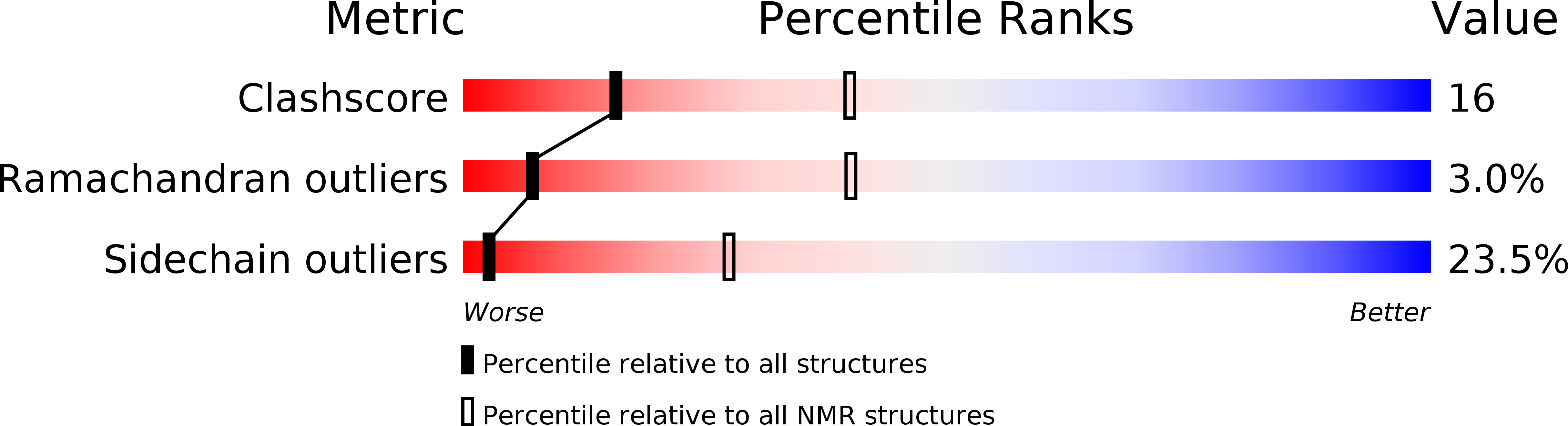Deposition Date
2014-08-04
Release Date
2015-07-01
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2MSK
Keywords:
Title:
Solution Structure and Chemical Shift Assignments for BeF3 activated Receiver Domain of Nitrogen Regulatory Protein C (NtrC) at 35C
Biological Source:
Source Organism(s):
Salmonella typhimurium (Taxon ID: 990282)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
300
Conformers Submitted:
25
Selection Criteria:
structures with the lowest energy