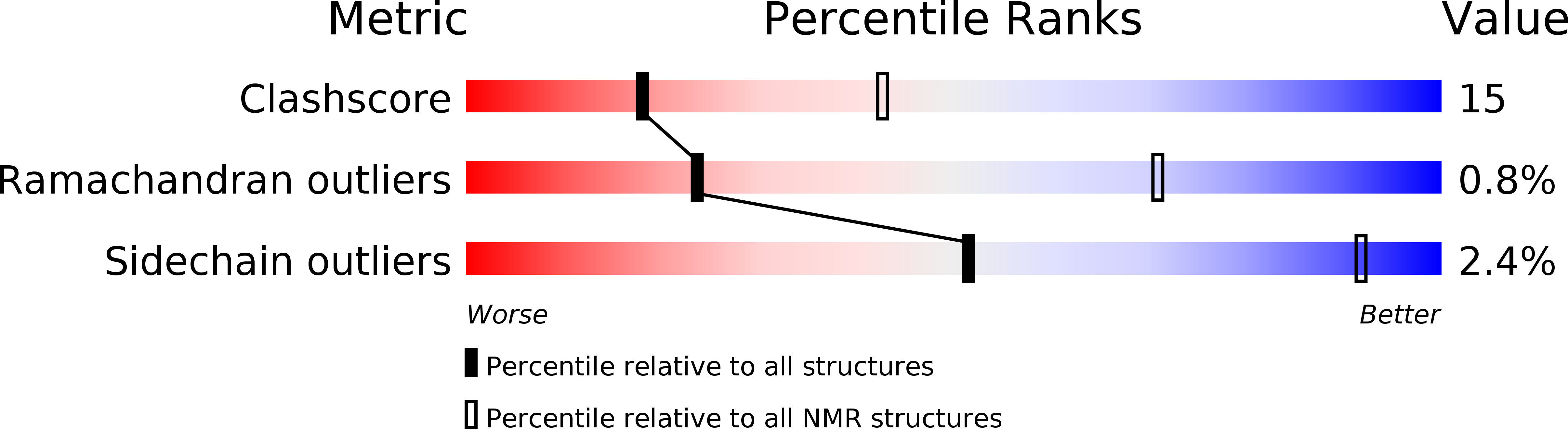
Deposition Date
2014-04-05
Release Date
2015-03-18
Last Version Date
2024-05-01
Entry Detail
PDB ID:
2MNH
Keywords:
Title:
Refined structure of outer membrane protein x in nanodisc by measuring residual dipolar couplings
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 1403831)
Expression System(s):
Method Details:
Experimental Method:
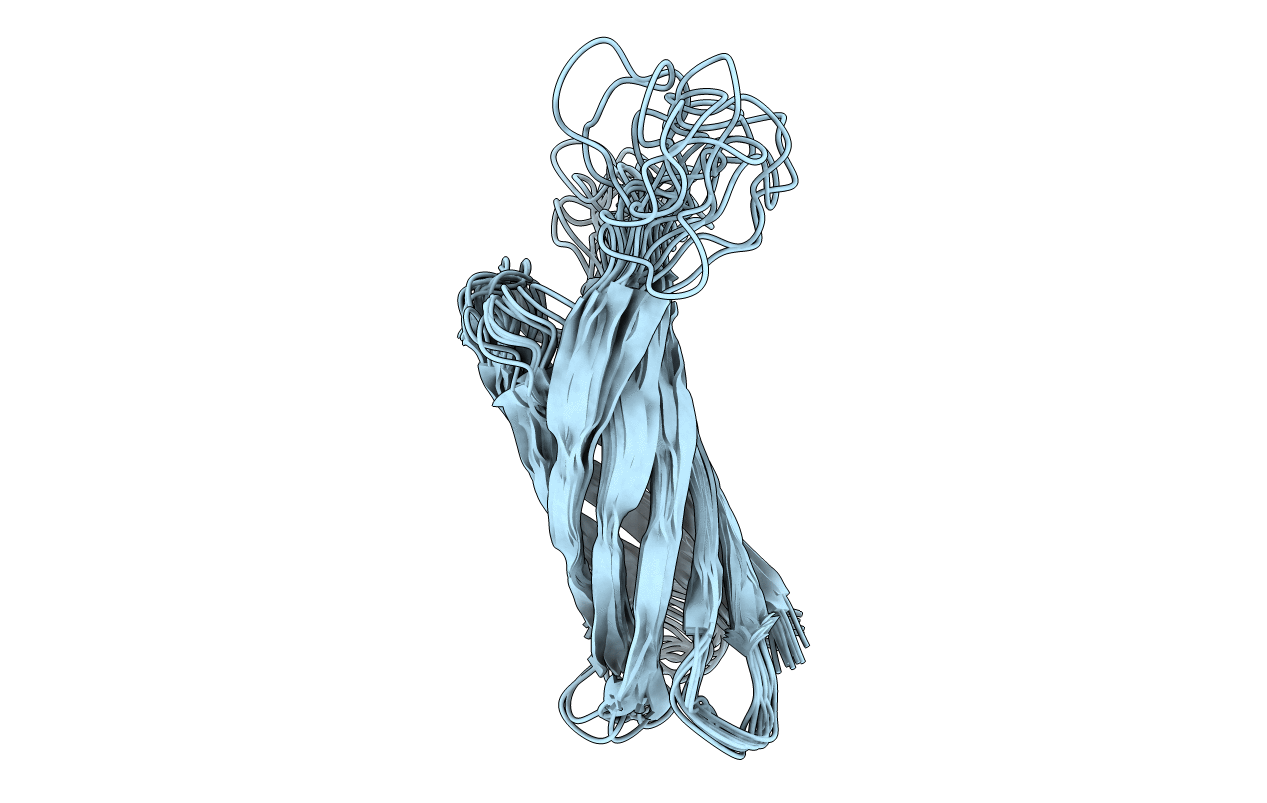
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the least restraint violations